Neurotrophins inhibit major histocompatibility class II inducibility of microglia: involvement of the p75 neurotrophin receptor
- PMID: 9576961
- PMCID: PMC20456
- DOI: 10.1073/pnas.95.10.5779
Neurotrophins inhibit major histocompatibility class II inducibility of microglia: involvement of the p75 neurotrophin receptor
Abstract
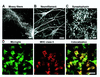
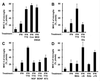
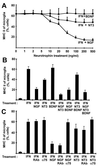
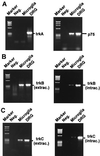
Major histocompatibility complex (MHC) molecules are rare in the healthy brain tissue, but are heavily expressed on microglial cells after inflammatory or neurodegenerative processes. We studied the conditions leading to the induction of MHC class II molecules in microglia by using explant cultures of neonatal rat hippocampus, a model of interacting neuronal networks. Interferon-gamma (IFN-gamma)-dependent MHC class II inducibility in microglia cells was very low, but strongly increased in the hippocampal slices after the blockade of neuronal activity by neurotoxins [tetrodotoxin (TTX), omega-conotoxin] or glutamate antagonists. None of these agents acted directly on isolated microglia cells. We found that neurotrophins modulate microglial MHC class II expression. MHC class II inducibility was enhanced by neutralization of neurotrophins produced locally within the cultured tissues and was inhibited by the addition of nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), or neurotrophin-3 (NT3). NGF and, to a lower extent, NT3 acted directly on isolated microglia via the p75 neurotrophin receptor and inhibited MHC class II inducibility as shown by blockade of the p75 neurotrophin receptor with antibodies. Our data suggest that neurotrophins secreted by electrically active neurons control the antigen-presenting potential of microglia cells, and indicate that this effect is mediated partly via the p75 neurotrophin receptor.
Figures

References
-
- Wekerle H, Linington C, Lassmann H, Meyermann R. Trends Neurosci. 1986;9:271–277.
-
- Itagaki S, McGeer P L, Akiyama H. Neurosci Lett. 1988;91:259–264. - PubMed
-
- Perlmutter L S, Scott S A, Chui H C. J Neurosci Res. 1992;33:549–558. - PubMed
-
- McGeer P L, Itagaki S, McGeer E G. Acta Neuropathol. 1988;76:550–557. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

