Treatment of hepatocellular carcinoma with octreotide: a randomised controlled study
- PMID: 9577356
- PMCID: PMC1727020
- DOI: 10.1136/gut.42.3.442
Treatment of hepatocellular carcinoma with octreotide: a randomised controlled study
Abstract
Background: Standard treatment of inoperable hepatocellular carcinoma has not been established. Somatostatin has been shown to possess antimitotic activity against a variety of non-endocrine tumours.
Aims: To assess the presence of somatostatin receptors in human liver and to treat advanced hepatocellular carcinoma with the somatostatin analogue, octreotide.
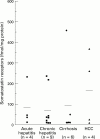
Methods: Somatostatin receptors were measured in liver tissue homogenates from patients with acute and chronic hepatitis, cirrhosis, and hepatocellular carcinoma. Fifty eight patients with advanced hepatocellular carcinoma were randomised to receive either subcutaneous octreotide 250 micrograms twice daily, or no treatment. Groups were comparable with respect to age, sex, Okuda classification, presence of cirrhosis, and liver biochemistry and virology.
Results: Various amounts of somatostatin receptors were identified in liver tissue of all patients including those with hepatocellular carcinoma. Treated patients had an increased median survival (13 months versus four months, p = 0.002, log rank test) and an increased cumulative survival rate at six and 12 months (75% versus 37%, and 56% versus 13% respectively). Octreotide administration significantly reduced alpha fetoprotein levels at six months. When a multivariable Cox's proportional hazards model was fitted, variables associated with increased survival were: treatment administration, absence of cirrhosis, increased serum albumin, and small tumours. Treated patients clearly had a lower hazard (0.383) in the multivariate analysis.
Conclusions: Octreotide administration significantly improves survival and is a valuable alternative in the treatment of inoperable hepatocellular carcinoma.
Figures



Comment in
-
Octreotide in hepatocellular carcinoma.Gut. 1998 Mar;42(3):316-7. doi: 10.1136/gut.42.3.316. Gut. 1998. PMID: 9577332 Free PMC article. No abstract available.
-
Complete regression of advanced HCC with long acting octreotide.Gut. 2003 Oct;52(10):1531. doi: 10.1136/gut.52.10.1531-a. Gut. 2003. PMID: 12970151 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
