Characterization of IS1515, a functional insertion sequence in Streptococcus pneumoniae
- PMID: 9580131
- PMCID: PMC107034
- DOI: 10.1128/JB.180.6.1381-1388.1998
Characterization of IS1515, a functional insertion sequence in Streptococcus pneumoniae
Abstract
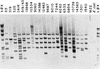
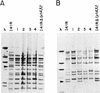
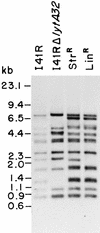
We describe the characterization of a new insertion sequence, IS1515, identified in the genome of Streptococcus pneumoniae I41R, an unencapsulated mutant isolated many years ago (R. Austrian, H. P. Bernheimer, E. E. B. Smith, and G. T. Mills, J. Exp. Med. 110:585-602, 1959). A copy of this element located in the cap1EI41R gene was sequenced. The 871-bp-long IS1515 element possesses 12-bp perfect inverted repeats and generates a 3-bp target duplication upon insertion. The IS encodes a protein of 271 amino acid residues similar to the putative transposases of other insertion sequences, namely IS1381 from S. pneumoniae, ISL2 from Lactobacillus helveticus, IS702 from the cyanobacterium Calothrix sp. strain PCC 7601, and IS112 from Streptomyces albus G. IS1515 appears to be present in the genome of most type 1 pneumococci in a maximum of 13 copies, although it has also been found in the chromosome of pneumococcal isolates belonging to other serotypes. We have found that the unencapsulated phenotype of strain 141R is the result of both the presence of an IS1515 copy and a frameshift mutation in the cap1EI41R gene. Precise excision of the IS was observed in the type 1 encapsulated transformants isolated in experiments designed to repair the frameshift. These results reveal that IS1515 behaves quite differently from other previously described pneumococcal insertion sequences. Several copies of IS1515 were also able to excise and move to another locations in the chromosome of S. pneumoniae. To our knowledge, this is the first report of a functional IS in pneumococcus.
Figures



Similar articles
-
Identification and characterization of IS1381, a new insertion sequence in Streptococcus pneumoniae.J Bacteriol. 1997 Apr;179(7):2459-63. doi: 10.1128/jb.179.7.2459-2463.1997. J Bacteriol. 1997. PMID: 9079939 Free PMC article.
-
Characterization of IS1167, a new insertion sequence in Streptococcus pneumoniae.Plasmid. 1995 Mar;33(2):127-38. doi: 10.1006/plas.1995.1014. Plasmid. 1995. PMID: 7597107
-
Isolation, characterization, and nucleotide sequence of IS1202, an insertion sequence of Streptococcus pneumoniae.J Bacteriol. 1994 Jul;176(14):4437-43. doi: 10.1128/jb.176.14.4437-4443.1994. J Bacteriol. 1994. PMID: 8021229 Free PMC article.
-
Transposition mechanisms and biotechnology applications of the medaka fish Tol2 transposable element.Adv Biophys. 2004;38:161-80. Adv Biophys. 2004. PMID: 15493333 Review.
-
Genomics and Genetics of Streptococcus pneumoniae.Microbiol Spectr. 2019 May;7(3):10.1128/microbiolspec.gpp3-0025-2018. doi: 10.1128/microbiolspec.GPP3-0025-2018. Microbiol Spectr. 2019. PMID: 31111814 Free PMC article. Review.
Cited by
-
A new pneumococcal serotype, 11E, has a variably inactivated wcjE gene.J Infect Dis. 2010 Jul 1;202(1):29-38. doi: 10.1086/653123. J Infect Dis. 2010. PMID: 20507232 Free PMC article.
-
Characterization of Streptococcus suis genes encoding proteins homologous to sortase of gram-positive bacteria.J Bacteriol. 2002 Feb;184(4):971-82. doi: 10.1128/jb.184.4.971-982.2002. J Bacteriol. 2002. PMID: 11807057 Free PMC article.
-
Insertion sequence 1515 in the ply gene of a type 1 clinical isolate of Streptococcus pneumoniae abolishes pneumolysin expression.J Clin Microbiol. 2007 Jul;45(7):2296-7. doi: 10.1128/JCM.02168-06. Epub 2007 May 9. J Clin Microbiol. 2007. PMID: 17494718 Free PMC article.
-
Chromosomal context directs high-frequency precise excision of IS492 in Pseudoalteromonas atlantica.Proc Natl Acad Sci U S A. 2007 Feb 6;104(6):1901-6. doi: 10.1073/pnas.0608633104. Epub 2007 Jan 30. Proc Natl Acad Sci U S A. 2007. PMID: 17264213 Free PMC article.
-
Predicted functions and linkage specificities of the products of the Streptococcus pneumoniae capsular biosynthetic loci.J Bacteriol. 2007 Nov;189(21):7856-76. doi: 10.1128/JB.00837-07. Epub 2007 Aug 31. J Bacteriol. 2007. PMID: 17766420 Free PMC article.
References
-
- Arrecubieta C, García E, López R. Sequence and transcriptional analysis of a DNA region involved in the production of capsular polysaccharide in Streptococcus pneumoniae type 3. Gene. 1995;167:1–7. - PubMed
-
- Craig N L. Transposition. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C: ASM Press; 1996. pp. 2339–2362.
-
- David F, De Céspedes G, Delbos F, Horaud T. Diversity of chromosomal genetic elements and a gene identification in antibiotic-resistant strains of Streptococcus pneumoniae and Streptococcus bovis. Plasmid. 1993;29:147–153. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

