A homogeneous, ligase-mediated DNA diagnostic test
- PMID: 9582198
- PMCID: PMC310725
- DOI: 10.1101/gr.8.5.549
A homogeneous, ligase-mediated DNA diagnostic test
Abstract
Single-nucleotide variations are the most widely distributed genetic markers in the human genome. A subset of these variations, the substitution mutations, are responsible for most genetic disorders. As single nucleotide polymorphism (SNP) markers are being developed for molecular diagnosis of genetic disorders and large-scale population studies for genetic analysis of complex traits, a simple, sensitive, and specific test for single nucleotide changes is highly desirable. In this report we describe the development of a homogeneous DNA detection method that requires no further manipulations after the initial reaction is set up. This assay, named dye-labeled oligonucleotide ligation (DOL), combines the PCR and the oligonucleotide ligation reaction in a two-stage thermal cycling sequence with fluorescence resonance energy transfer (FRET) detection monitored in real time. Because FRET occurs only when the donor and acceptor dyes are in close proximity, one can infer the genotype or mutational status of a DNA sample by monitoring the specific ligation of dye-labeled oligonucleotide probes. We have successfully applied the DOL assay to genotype 10 SNPs or mutations. By designing the PCR primers and ligation probes in a consistent manner, multiple assays can be done under the same thermal cycling conditions. The standardized design and execution of the DOL assay means that it can be automated for high-throughput genotyping in large-scale population studies.
Figures
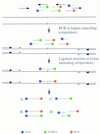

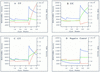

References
-
- Collins FS, Guyer MS, Chakravarti A. Variations on a theme: Cataloging human DNA sequence variation. Science. 1997;278:1580–1581. - PubMed
-
- Couch FJ, Weber BL. Mutations and polymorphisms in the familial early-onset breast cancer (BRCA1) gene. Hum Mutat. 1996;8:8–18. - PubMed
-
- Eggerding FA. A one-step coupled amplification and oligonucleotide ligation procedure for multiplex genetic typing. PCR Methods Applic. 1995;4:337–345. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
