Neuropharmacological characterization of basal forebrain cholinergic stimulated cataplexy in narcoleptic canines
- PMID: 9582257
- PMCID: PMC8848856
- DOI: 10.1006/exnr.1998.6787
Neuropharmacological characterization of basal forebrain cholinergic stimulated cataplexy in narcoleptic canines
Abstract
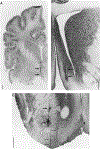
Basal forebrain (BF) cholinergic regulation of cataplexy was investigated in narcoleptic canines. Specific cholinergic agonists and antagonists, and excitatory or inhibitory amino acid neurotransmitter receptor agonists, were perfused through microdialysis probes implanted bilaterally in the BF of narcoleptic canines. Cataplexy was monitored using the food-elicited cataplexy test (FECT) and recordings of electroencephalogram, electrooculogram, and electromyogram. In narcoleptic canines, carbachol and oxotremorine (10(-5)-10(-3) M), but not McN-A-343 or nicotine (10(-4)-10(-3) M), produced a dose-dependent increase in cataplexy. In addition, N-methyl-d-aspartate (10(-4)-10(-3) M) and kainic acid (10(-5)-10(-4) M) did not have any effects, while muscimol (10(-3) M) produced a weak (P < 0.10) increase in cataplexy. In control canines, carbachol (10(-5)-10(-3) M), but not oxotremorine (10(-4)-10(-3) M), produced muscle atonia after the highest concentration in one of three animals. Carbachol (10(-3) M)-induced cataplexy in narcoleptic canines was blocked by equimolar perfusion with the muscarinic antagonists atropine, gallamine, and 4-DAMP but not pirenzepine. These findings indicate that carbachol-stimulated cataplexy in the BF of narcoleptic canines is mediated by M2, and perhaps M3, muscarinic receptors. The release of acetylcholine in the BF was also examined during FECT and non-FECT behavioral stimulation in narcoleptic and control canines. A significant increase in acetylcholine release was found in both narcoleptic and control BF during FECT stimulation. In contrast, simple motor activity and feeding, approximating that which occurs during an FECT, did not affect acetylcholine release in the BF of narcoleptic canines. These findings indicate that BF acetylcholine release is enhanced during learned emotion/reward associated behaviors in canines.
Copyright 1998 Academic Press.
Figures




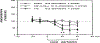

Similar articles
-
Cholinergic regulation of cataplexy in canine narcolepsy in the pontine reticular formation is mediated by M2 muscarinic receptors.Sleep. 1994 Aug;17(5):424-35. Sleep. 1994. PMID: 7991953 Free PMC article.
-
Cholinergic mechanisms in canine narcolepsy--I. Modulation of cataplexy via local drug administration into the pontine reticular formation.Neuroscience. 1994 Apr;59(3):511-22. doi: 10.1016/0306-4522(94)90173-2. Neuroscience. 1994. PMID: 8008205 Free PMC article.
-
Pharmacological characterization of the muscarinic receptor subtypes responsible for the contractile response in the rat urinary bladder.J Auton Pharmacol. 1997 Feb;17(1):21-5. doi: 10.1046/j.1365-2680.1997.00436.x. J Auton Pharmacol. 1997. PMID: 9201556
-
Effect of cholinergic drugs on the concentration of intracellular free calcium of rat pituitary intermediate lobe cells.Brain Res Bull. 1999 Sep 1;50(1):53-7. doi: 10.1016/s0361-9230(99)00085-4. Brain Res Bull. 1999. PMID: 10507472
-
Cholinergic mechanisms in canine narcolepsy--II. Acetylcholine release in the pontine reticular formation is enhanced during cataplexy.Neuroscience. 1994 Apr;59(3):523-30. doi: 10.1016/0306-4522(94)90174-0. Neuroscience. 1994. PMID: 8008206 Free PMC article.
Cited by
-
Hypocretin increases impulse flow in the septohippocampal GABAergic pathway: implications for arousal via a mechanism of hippocampal disinhibition.J Neurosci. 2002 Sep 1;22(17):7754-65. doi: 10.1523/JNEUROSCI.22-17-07754.2002. J Neurosci. 2002. PMID: 12196599 Free PMC article.
-
Fluctuation of primary motor cortex excitability during cataplexy in narcolepsy.Ann Clin Transl Neurol. 2019 Jan 20;6(2):210-221. doi: 10.1002/acn3.670. eCollection 2019 Feb. Ann Clin Transl Neurol. 2019. PMID: 30847354 Free PMC article.
-
Cataplexy--clinical aspects, pathophysiology and management strategy.Nat Rev Neurol. 2014 Jul;10(7):386-95. doi: 10.1038/nrneurol.2014.97. Epub 2014 Jun 3. Nat Rev Neurol. 2014. PMID: 24890646 Free PMC article. Review.
-
Intrinsic membrane properties and cholinergic modulation of mouse basal forebrain glutamatergic neurons in vitro.Neuroscience. 2017 Jun 3;352:249-261. doi: 10.1016/j.neuroscience.2017.04.002. Epub 2017 Apr 12. Neuroscience. 2017. PMID: 28411158 Free PMC article.
-
Role of orexin in modulating arousal, feeding, and motivation.Front Behav Neurosci. 2013 Apr 18;7:28. doi: 10.3389/fnbeh.2013.00028. eCollection 2013. Front Behav Neurosci. 2013. PMID: 23616752 Free PMC article.
References
-
- Armatruda T, Black D, McKenna T, McCarley RW, and Hobson JA. 1975. Sleep cycle control and cholinergic mechanisms: differential effects of carbachol injections at pontine brainstem sites. Brain Res. 98: 501–515. - PubMed
-
- Azuma S, Kodama T, Honda K, and Inoue S. 1996. State-dependent changes in extracellular glutamate in the medial preoptic area of freely behaving rats. Neurosci. Lett 214: 179–182. - PubMed
-
- Baker TL, and Dement WC. 1985. Canine narcolepsycataplexy syndrome: Evidence for an inherited monoaminergic-cholinergic imbalance. In Brain Mechanisms of Sleep (McGinty DJ, Drucker-Collin R, Morrison A, and Parmengiani P, Eds.), pp. 199–233. Raven Press, New York.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

