Roles of the tumor suppressor p53 and the cyclin-dependent kinase inhibitor p21WAF1/CIP1 in receptor-mediated apoptosis of WEHI 231 B lymphoma cells
- PMID: 9584145
- PMCID: PMC2212285
- DOI: 10.1084/jem.187.10.1671
Roles of the tumor suppressor p53 and the cyclin-dependent kinase inhibitor p21WAF1/CIP1 in receptor-mediated apoptosis of WEHI 231 B lymphoma cells
Abstract
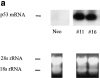
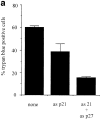
Treatment of WEHI 231 immature B lymphoma cells with an antibody against their surface immunoglobulin M (anti-IgM) induces apoptosis and has been studied extensively as a model of self-induced B cell tolerance. Since the tumor suppressor protein p53 has been implicated in apoptosis in a large number of cell types and has been found to be mutated in a variety of B cell tumors, here we sought to determine whether p53 and the p53 target gene cyclin-dependent kinase inhibitor p21(WAF1/CIP1) were involved in anti-IgM-induced cell death. Anti-IgM treatment of WEHI 231 cells increased expression of p53 and p21 protein levels. Ectopic expression of wild-type p53 in WEHI 231 cells induced both p21 expression and apoptosis. Ectopic expression of p21 similarly induced apoptosis. Rescue of WEHI 231 cells from apoptosis by costimulation with CD40 ligand ablated the increase in p21 expression. Lastly, a significant decrease in anti-IgM-mediated apoptosis was seen upon downregulation of endogenous p53 activity by expression of a dominant-negative p53 protein or upon microinjection of an antisense p21 expression vector or antibody. Taken together, the above data demonstrate important roles for p53 and p21 proteins in receptor-mediated apoptosis of WEHI 231 B cells.
Figures
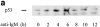
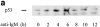






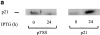
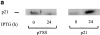
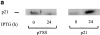


References
-
- Boyd AW, Schrader JW. The regulation of growth and differentiation of a murine B-cell lymphoma. II. The inhibition of WEHI 231 by anti-immunoglobulin antibodies. J Immunol. 1981;126:2466–2469. - PubMed
-
- Monroe JG, Seyfert VL. Studies of surface immunoglobulin-dependent B cell activation. Immunol Res. 1988;7:136–151. - PubMed
-
- Ralph P. Functional subsets of murine and human B lymphocyte cell lines. Immunol Rev. 1979;48:107–121. - PubMed
-
- Benhamou LE, Cazenave P-A, Sarthou P. Anti-immunoglobulins induce death by apoptosis in WEHI 231 B lymphoma cells. Eur J Immunol. 1990;20:1405–1407. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

