RACK1, a receptor for activated C kinase and a homolog of the beta subunit of G proteins, inhibits activity of src tyrosine kinases and growth of NIH 3T3 cells
- PMID: 9584165
- PMCID: PMC108906
- DOI: 10.1128/MCB.18.6.3245
RACK1, a receptor for activated C kinase and a homolog of the beta subunit of G proteins, inhibits activity of src tyrosine kinases and growth of NIH 3T3 cells
Abstract
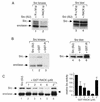
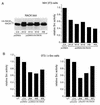
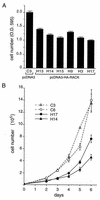
To isolate and characterize proteins that interact with the unique domain and SH3 and SH2 domains of Src and potentially regulate Src activity, we used the yeast two-hybrid assay to screen a human lung fibroblast cDNA library. We identified RACK1, a receptor for activated C kinase and a homolog of the beta subunit of G proteins, as a Src-binding protein. Using GST-Src fusion proteins, we determined that RACK1 binds to the SH2 domain of Src. Coimmunoprecipitation of Src and RACK1 was demonstrated with NIH 3T3 cells. Purified GST-RACK1 inhibited the in vitro kinase activity of Src in a concentration-dependent manner. GST-RACK1 (2 microM) inhibited the activities of purified Src and Lck tyrosine kinases by 40 to 50% but did not inhibit the activities of three serine/threonine kinases that we tested. Tyrosine phosphorylation on many cellular proteins decreased in 293T cells that transiently overexpressed RACK1. Src activity and cell growth rates decreased by 40 to 50% in NIH 3T3 cells that stably overexpressed RACK1. Flow cytometric analyses revealed that RACK1-overexpressing cells do not show an increased rate of necrosis or apoptosis but do spend significantly more time in G0/G1 than do wild-type cells. Prolongation of G0/G1 could account for the increased doubling time of RACK1-overexpressing cells. We suggest that RACK1 exerts its effect on the NIH 3T3 cell cycle in part by inhibiting Src activity.
Figures

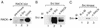
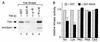

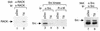
References
-
- Allen B G, Katz S. Isolation and characterization of the calcium and phospholipid dependent protein kinase (protein kinase C) subtypes from bovine heart. Biochemistry. 1991;30:4334–4340. - PubMed
-
- Altmeyer A, Simmons R C, Krajewski S, Reed J C, Bornkamm G W, Chen-Kiang S. Reversal of EBV immortalization precedes apoptosis in IL-6 induced human B cell terminal differentiation. Immunity. 1997;7:667–677. - PubMed
-
- Bartel P L, Chien C T, Stemglanz R, Fields S. Using the two hybrid system to detect protein-protein interactions. In: Hartley D A, editor. Development: a practical approach. Oxford, England: Oxford University Press; 1993. pp. 153–179.
-
- Bohen S P, Kralli A, Yamamoto K R. Hold’em and fold’em: chaperones and signal transduction. Science. 1995;268:1303–1304. - PubMed
-
- Bolen J B, Thiele C J, Israel M A, Yonemoto W, Lipsich L A, Brugge J S. Enhancement of cellular src gene product associated tyrosyl kinase activity following polyoma virus infection and transformation. Cell. 1984;38:767–777. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
