Genetic and biochemical analysis of p23 and ansamycin antibiotics in the function of Hsp90-dependent signaling proteins
- PMID: 9584173
- PMCID: PMC108914
- DOI: 10.1128/MCB.18.6.3330
Genetic and biochemical analysis of p23 and ansamycin antibiotics in the function of Hsp90-dependent signaling proteins
Abstract
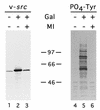
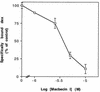
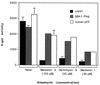
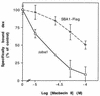
The ubiquitous molecular chaperone Hsp90 acts in concert with a cohort of associated proteins to facilitate the functional maturation of a number of cellular signaling proteins, such as steroid hormone receptors and oncogene tyrosine kinases. The Hsp90-associated protein p23 is required for the assembly of functional steroid aporeceptor complexes in cell lysates, and Hsp90-binding ansamycin antibiotics disrupt the activity of Hsp90-dependent signaling proteins in cultured mammalian cells and prevent the association of p23 with Hsp90-receptor heterocomplexes; these observations have led to the hypotheses that p23 is required for the maturation of Hsp90 target proteins and that ansamycin antibiotics abrogate the activity of such proteins by disrupting the interaction of p23 with Hsp90. In this study, I demonstrate that ansamycin antibiotics disrupt the function of Hsp90 target proteins expressed in yeast cells; prevent the assembly of Sba1, a yeast p23-like protein, into steroid receptor-Hsp90 complexes; and result in the assembly of receptor-Hsp90 complexes that are defective for ligand binding. To assess the role of p23 in Hsp90 target protein function, I show that the activity of Hsp90 target proteins is unaffected by deletion of SBA1. Interestingly, steroid receptor activity in cells lacking Sba1 displays increased sensitivity to ansamycin antibiotics, and this phenotype is rescued by the expression of human p23 in yeast cells. These findings indicate that Hsp90-dependent signaling proteins can achieve a functional conformation in vivo in the absence of p23. Furthermore, while the presence of p23 decreases the sensitivity of Hsp90-dependent processes to ansamycin treatment, ansamycin antibiotics disrupt signaling through some mechanism other than altering the Hsp90-p23 interaction.
Figures





References
-
- Bohen S P. Hsp90 mutants disrupt glucocorticoid receptor ligand binding and destabilize aporeceptor complexes. J Biol Chem. 1995;270:29433–29438. - PubMed
-
- Bohen, S. P. Unpublished observations.
-
- Bohen S P, Kralli A, Yamamoto K R. Hold ’em and fold ’em: chaperones and signal transduction. Science. 1995;268:1303–1304. . (Comment.) - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
