Nuclear localization of cyclin B1 controls mitotic entry after DNA damage
- PMID: 9585407
- PMCID: PMC2132764
- DOI: 10.1083/jcb.141.4.875
Nuclear localization of cyclin B1 controls mitotic entry after DNA damage
Abstract
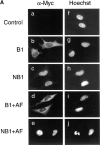
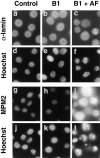
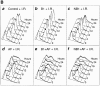
Mitosis in human cells is initiated by the protein kinase Cdc2-cyclin B1, which is activated at the end of G2 by dephosphorylation of two inhibitory residues, Thr14 and Tyr15. The G2 arrest that occurs after DNA damage is due in part to stabilization of phosphorylation at these sites. We explored the possibility that entry into mitosis is also regulated by the subcellular location of Cdc2-cyclin B1, which is suddenly imported into the nucleus at the end of G2. We measured the timing of mitosis in HeLa cells expressing a constitutively nuclear cyclin B1 mutant. Parallel studies were performed with cells expressing Cdc2AF, a Cdc2 mutant that cannot be phosphorylated at inhibitory sites. Whereas nuclear cyclin B1 and Cdc2AF each had little effect under normal growth conditions, together they induced a striking premature mitotic phenotype. Nuclear targeting of cyclin B1 was particularly effective in cells arrested in G2 by DNA damage, where it greatly reduced the damage-induced G2 arrest. Expression of nuclear cyclin B1 and Cdc2AF also resulted in significant defects in the exit from mitosis. Thus, nuclear targeting of cyclin B1 and dephosphorylation of Cdc2 both contribute to the control of mitotic entry and exit in human cells.
Figures










Similar articles
-
Role of inhibitory CDC2 phosphorylation in radiation-induced G2 arrest in human cells.J Cell Biol. 1996 Aug;134(4):963-70. doi: 10.1083/jcb.134.4.963. J Cell Biol. 1996. PMID: 8769420 Free PMC article.
-
Centrosomal and cytoplasmic Cdc2/cyclin B1 activation precedes nuclear mitotic events.Exp Cell Res. 2000 May 25;257(1):11-21. doi: 10.1006/excr.2000.4872. Exp Cell Res. 2000. PMID: 10854050
-
The role of Cdc2 feedback loop control in the DNA damage checkpoint in mammalian cells.Cancer Res. 1997 Nov 15;57(22):5168-78. Cancer Res. 1997. PMID: 9371520
-
Regulation of the G2/M transition by p53.Oncogene. 2001 Apr 5;20(15):1803-15. doi: 10.1038/sj.onc.1204252. Oncogene. 2001. PMID: 11313928 Review.
-
Cyclin B1 and CDK1: nuclear localization and upstream regulators.Prog Cell Cycle Res. 2003;5:335-47. Prog Cell Cycle Res. 2003. PMID: 14593728 Review.
Cited by
-
Ubiquitin-specific protease 22 is a deubiquitinase of CCNB1.Cell Discov. 2015;1:15028-. doi: 10.1038/celldisc.2015.28. Epub 2015 Oct 13. Cell Discov. 2015. PMID: 27030811 Free PMC article.
-
Therapeutic targeting of Chk1 in NSCLC stem cells during chemotherapy.Cell Death Differ. 2012 May;19(5):768-78. doi: 10.1038/cdd.2011.170. Epub 2011 Nov 25. Cell Death Differ. 2012. PMID: 22117197 Free PMC article.
-
Activation of Cdh1-dependent APC is required for G1 cell cycle arrest and DNA damage-induced G2 checkpoint in vertebrate cells.EMBO J. 2001 Nov 15;20(22):6499-508. doi: 10.1093/emboj/20.22.6499. EMBO J. 2001. PMID: 11707420 Free PMC article.
-
MicroRNA-144 inhibits cell proliferation, migration and invasion in human hepatocellular carcinoma by targeting CCNB1.Cancer Cell Int. 2019 Jan 14;19:15. doi: 10.1186/s12935-019-0729-x. eCollection 2019. Cancer Cell Int. 2019. PMID: 30651720 Free PMC article.
-
Therapeutic Potency of Nanoformulations of siRNAs and shRNAs in Animal Models of Cancers.Pharmaceutics. 2018 May 26;10(2):65. doi: 10.3390/pharmaceutics10020065. Pharmaceutics. 2018. PMID: 29861465 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

