Ganglioside structure dictates signal transduction by cholera toxin and association with caveolae-like membrane domains in polarized epithelia
- PMID: 9585411
- PMCID: PMC2132772
- DOI: 10.1083/jcb.141.4.917
Ganglioside structure dictates signal transduction by cholera toxin and association with caveolae-like membrane domains in polarized epithelia
Abstract
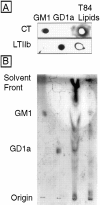
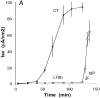
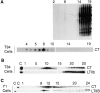
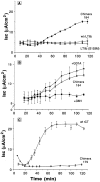
In polarized cells, signal transduction by cholera toxin (CT) requires apical endocytosis and retrograde transport into Golgi cisternae and perhaps ER (Lencer, W.I., C. Constable, S. Moe, M. Jobling, H.M. Webb, S. Ruston, J.L. Madara, T. Hirst, and R. Holmes. 1995. J. Cell Biol. 131:951-962). In this study, we tested whether CT's apical membrane receptor ganglioside GM1 acts specifically in toxin action. To do so, we used CT and the related Escherichia coli heat-labile type II enterotoxin LTIIb. CT and LTIIb distinguish between gangliosides GM1 and GD1a at the cell surface by virtue of their dissimilar receptor-binding B subunits. The enzymatically active A subunits, however, are homologous. While both toxins bound specifically to human intestinal T84 cells (Kd approximately 5 nM), only CT elicited a cAMP-dependent Cl- secretory response. LTIIb, however, was more potent than CT in eliciting a cAMP-dependent response from mouse Y1 adrenal cells (toxic dose 10 vs. 300 pg/well). In T84 cells, CT fractionated with caveolae-like detergent-insoluble membranes, but LTIIb did not. To investigate further the relationship between the specificity of ganglioside binding and partitioning into detergent-insoluble membranes and signal transduction, CT and LTIIb chimeric toxins were prepared. Analysis of these chimeric toxins confirmed that toxin-induced signal transduction depended critically on the specificity of ganglioside structure. The mechanism(s) by which ganglioside GM1 functions in signal transduction likely depends on coupling CT with caveolae or caveolae-related membrane domains.
Figures
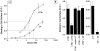

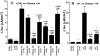
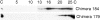
References
-
- Anderson RG. Plasmalemmal caveolae and GPI-anchored membrane proteins. Curr Opin Cell Biol. 1993b;5:647–652. - PubMed
-
- Anderson RG, Kamen BA, Rothberg KG, Lacey SW. Potocytosis: sequestration and transport of small molecules by caveolae. Science. 1992;255:410–411. - PubMed
-
- Bhatnagar RS, Gordon JI. Understanding covalent modifications of proteins by lipids: where cell biology and biophysics merge. Trends Cell Biol. 1997;7:14–20. - PubMed
-
- Bretscher MS, Munro S. Cholesterol and the Golgi apparatus. Science. 1993;261:1280–1281. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI031940/AI/NIAID NIH HHS/United States
- DK 48106/DK/NIDDK NIH HHS/United States
- R01 DK053056/DK/NIDDK NIH HHS/United States
- P01 DK033506/DK/NIDDK NIH HHS/United States
- R37 DK035932/DK/NIDDK NIH HHS/United States
- R01 DK048106/DK/NIDDK NIH HHS/United States
- DK/AI 53056/DK/NIDDK NIH HHS/United States
- P30 DK034854/DK/NIDDK NIH HHS/United States
- T32 DK007477/DK/NIDDK NIH HHS/United States
- T32-DK07477/DK/NIDDK NIH HHS/United States
- R01 AI014107/AI/NIAID NIH HHS/United States
- R37 DK048106/DK/NIDDK NIH HHS/United States
- R56 DK053056/DK/NIDDK NIH HHS/United States
- R01 AI053056/AI/NIAID NIH HHS/United States
- R37 AI014107/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

