Lipid domain structure of the plasma membrane revealed by patching of membrane components
- PMID: 9585412
- PMCID: PMC2132776
- DOI: 10.1083/jcb.141.4.929
Lipid domain structure of the plasma membrane revealed by patching of membrane components
Abstract
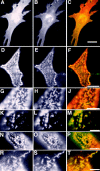
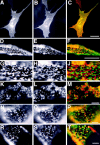
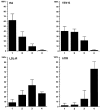
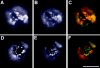
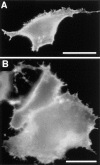
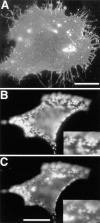
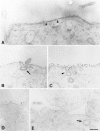
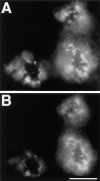
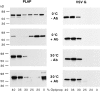
Lateral assemblies of glycolipids and cholesterol, "rafts," have been implicated to play a role in cellular processes like membrane sorting, signal transduction, and cell adhesion. We studied the structure of raft domains in the plasma membrane of non-polarized cells. Overexpressed plasma membrane markers were evenly distributed in the plasma membrane. We compared the patching behavior of pairs of raft markers (defined by insolubility in Triton X-100) with pairs of raft/non-raft markers. For this purpose we cross-linked glycosyl-phosphatidylinositol (GPI)-anchored proteins placental alkaline phosphatase (PLAP), Thy-1, influenza virus hemagglutinin (HA), and the raft lipid ganglioside GM1 using antibodies and/or cholera toxin. The patches of these raft markers overlapped extensively in BHK cells as well as in Jurkat T-lymphoma cells. Importantly, patches of GPI-anchored PLAP accumulated src-like protein tyrosine kinase fyn, which is thought to be anchored in the cytoplasmic leaflet of raft domains. In contrast patched raft components and patches of transferrin receptor as a non-raft marker were sharply separated. Taken together, our data strongly suggest that coalescence of cross-linked raft elements is mediated by their common lipid environments, whereas separation of raft and non-raft patches is caused by the immiscibility of different lipid phases. This view is supported by the finding that cholesterol depletion abrogated segregation. Our results are consistent with the view that raft domains in the plasma membrane of non-polarized cells are normally small and highly dispersed but that raft size can be modulated by oligomerization of raft components.
Figures
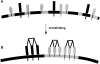
References
-
- Ahmed SN, Brown DA, London E. On the origin of sphingolipid/ cholesterol-rich detergent-insoluble cell membranes: physiological concentrations of cholesterol and sphingolipid induce formation of a detergent insoluble, liquid ordered lipid phase in model membranes. Biochemistry. 1997;36:10944–10953. - PubMed
-
- Brown DA. Interactions between GPI-anchored proteins and membrane lipids. Trends Cell Biol. 1992;2:338–343. - PubMed
-
- Brown DA. The tyrosine kinase connection: how GPI-anchored proteins activate T cells. Curr Opin Immunol. 1993;5:349–354. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

