Modulation of integrin activity is vital for morphogenesis
- PMID: 9585424
- PMCID: PMC2132760
- DOI: 10.1083/jcb.141.4.1073
Modulation of integrin activity is vital for morphogenesis
Abstract
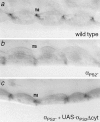
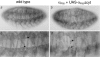
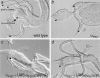
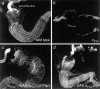
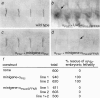
Cells can vary their adhesive properties by modulating the affinity of integrin receptors. The activation and inactivation of integrins by inside-out mechanisms acting on the cytoplasmic domains of the integrin subunits has been demonstrated in platelets, lymphocytes, and keratinocytes. We show that in the embryo, normal morphogenesis requires the alpha subunit cytoplasmic domain to control integrin adhesion at the right times and places. PS2 integrin (alphaPS2betaPS) adhesion is normally restricted to the muscle termini, where it is required for attaching the muscles to the ends of other muscles and to specialized epidermal cells. Replacing the wild-type alphaPS2 with mutant forms containing cytoplasmic domain deletions results in the rescue of the majority of defects associated with the absence of the alphaPS2 subunit, however, the mutant PS2 integrins are excessively active. Muscles containing these mutant integrins make extra muscle attachments at aberrant positions on the muscle surface, disrupting the muscle pattern and causing embryonic lethality. A gain- of-function phenotype is not observed in the visceral mesoderm, showing that regulation of integrin activity is tissue-specific. These results suggest that the alphaPS2 subunit cytoplasmic domain is required for inside-out regulation of integrin affinity, as has been seen with the integrin alphaIIbbeta3.
Figures

Similar articles
-
Requirements for the cytoplasmic domain of the alphaPS1, alphaPS2 and betaPS integrin subunits during Drosophila development.Development. 1998 Feb;125(4):701-11. doi: 10.1242/dev.125.4.701. Development. 1998. PMID: 9435290
-
PS2 integrin requirements in Drosophila embryo and wing morphogenesis.Dev Biol. 1993 May;157(1):49-59. doi: 10.1006/dbio.1993.1111. Dev Biol. 1993. PMID: 8482419
-
The PS integrins are required for a regulatory event during Drosophila wing morphogenesis.Ann N Y Acad Sci. 1998 Oct 23;857:99-109. doi: 10.1111/j.1749-6632.1998.tb10110.x. Ann N Y Acad Sci. 1998. PMID: 9917835 Review.
-
Specificity of PS integrin function during embryogenesis resides in the alpha subunit extracellular domain.EMBO J. 1997 Jul 16;16(14):4184-93. doi: 10.1093/emboj/16.14.4184. EMBO J. 1997. PMID: 9250662 Free PMC article.
-
Role of the PS integrins in Drosophila development.Immunol Cell Biol. 1995 Dec;73(6):558-64. doi: 10.1038/icb.1995.89. Immunol Cell Biol. 1995. PMID: 8713479 Review.
Cited by
-
Identification of integrin beta subunit mutations that alter heterodimer function in situ.Mol Biol Cell. 2004 Aug;15(8):3829-40. doi: 10.1091/mbc.e04-02-0085. Epub 2004 Jun 11. Mol Biol Cell. 2004. PMID: 15194810 Free PMC article.
-
PS integrins and laminins: key regulators of cell migration during Drosophila embryogenesis.PLoS One. 2011;6(9):e23893. doi: 10.1371/journal.pone.0023893. Epub 2011 Sep 16. PLoS One. 2011. PMID: 21949686 Free PMC article.
-
Vestigial is required during late-stage muscle differentiation in Drosophila melanogaster embryos.Mol Biol Cell. 2010 Oct 1;21(19):3304-16. doi: 10.1091/mbc.E10-04-0364. Epub 2010 Aug 4. Mol Biol Cell. 2010. PMID: 20685961 Free PMC article.
-
Integrins are required for cardioblast polarisation in Drosophila.BMC Dev Biol. 2012 Feb 21;12:8. doi: 10.1186/1471-213X-12-8. BMC Dev Biol. 2012. PMID: 22353787 Free PMC article.
-
LanB1 Cooperates With Kon-Tiki During Embryonic Muscle Migration in Drosophila.Front Cell Dev Biol. 2022 Jan 3;9:749723. doi: 10.3389/fcell.2021.749723. eCollection 2021. Front Cell Dev Biol. 2022. PMID: 35047493 Free PMC article.
References
-
- Adams JC, Watt FM. Changes in keratinocyte adhesion during terminal differentiation: reduction in fibronectin binding precedes α5β1integrin loss from the cell surface. Cell. 1990;63:425–435. - PubMed
-
- Arnaout MA. Structure and function of the leukocyte adhesion molecules CD11/CD18. Blood. 1990;75:1037–1050. - PubMed
-
- Babrant MC, Brower DL. PS2 Integrin requirements in Drosophilaembryo and wing morphogenesis. Dev Biol. 1993;157:49–59. - PubMed
-
- Bate M. The embryonic development of larval muscles in Drosophila. . Development. 1990;110:791–804. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

