Ultrabithorax regulates genes at several levels of the wing-patterning hierarchy to shape the development of the Drosophila haltere
- PMID: 9585507
- PMCID: PMC316835
- DOI: 10.1101/gad.12.10.1474
Ultrabithorax regulates genes at several levels of the wing-patterning hierarchy to shape the development of the Drosophila haltere
Abstract
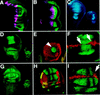
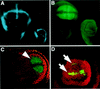
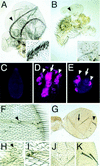
Arthropods and vertebrates are constructed of many serially homologous structures whose individual patterns are regulated by Hox genes. The Hox-regulated target genes and developmental pathways that determine the morphological differences between any homologous structures are not known. The differentiation of the Drosophila haltere from the wing through the action of the Ultrabithorax (Ubx) gene is a classic example of Hox regulation of serial homology, although no Ubx-regulated genes in the haltere have been identified previously. Here, we show that Ubx represses the expression of the Wingless (Wg) signaling protein and a subset of Wg- and Decapentaplegic-activated genes such as spalt-related, vestigial, Serum Response Factor, and achaete-scute, whose products regulate morphological features that differ between the wing and haltere. In addition, we found that some genes in the same developmental pathway are independently regulated by Ubx. Our results suggest that Ubx, and Hox genes in general, independently and selectively regulate genes that act at many levels of regulatory hierarchies to shape the differential development of serially homologous structures.
Figures


References
-
- Beachy PA, Helfand SL, Hogness DS. Segmental distribution of bithorax complex proteins during Drosophila development. Nature. 1985;313:545–551. - PubMed
-
- Brand A, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. - PubMed
-
- Burke R, Basler K. Hedgehog signaling in Drosophila eye and limb development-conserved machinery, divergent roles? Curr Opin Neurobiol. 1997;7:55–61. - PubMed
-
- Cabrera C, Botas J, Garcia-Bellido A. Distribution of Ultrabithorax proteins in mutants of Drosophila bithorax complex and its transregulatory genes. Nature. 1985;318:569–571.
-
- Carroll S. Homeotic genes and the evolution of arthropods and chordates. Review. Nature. 1995;376:479–485. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
