pavarotti encodes a kinesin-like protein required to organize the central spindle and contractile ring for cytokinesis
- PMID: 9585508
- PMCID: PMC316841
- DOI: 10.1101/gad.12.10.1483
pavarotti encodes a kinesin-like protein required to organize the central spindle and contractile ring for cytokinesis
Abstract
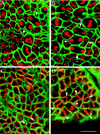
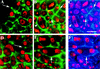
Mutations in the Drosophila gene pavarotti result in the formation of abnormally large cells in the embryonic nervous system. In mitotic cycle 16, cells of pav mutant embryos undergo normal anaphase but then develop an abnormal telophase spindle and fail to undertake cytokinesis. We show that the septin Peanut, actin, and the actin-associated protein Anillin, do not become correctly localized in pav mutants. pav encodes a kinesin-like protein, PAV-KLP, related to the mammalian MKLP-1. In cellularized embryos, the protein is localized to centrosomes early in mitosis, and to the midbody region of the spindle in late anaphase and telophase. We show that Polo kinase associates with PAV-KLP with which it shows an overlapping pattern of subcellular localization during the mitotic cycle and this distribution is disrupted in pav mutants. We suggest that PAV-KLP is required both to establish the structure of the telophase spindle to provide a framework for the assembly of the contractile ring, and to mobilize mitotic regulator proteins.
Figures






References
-
- Andreassen PR, Palmer DK, Wener MH, Margolis RL. Telophase disc: A new mammalian mitotic organelle that bisects telophase cells with a possible function in cytokinesis. J Cell Sci. 1991;99:523–534. - PubMed
-
- Bonaccorsi S, Giansanti MG, Gatti M. Asterless, a Drosophila gene specifically required for the formation of asters during male meiosis. Mol Biol Cell. 1996;7S:574a.
-
- Brown NH, Kafatos FC. Functional cDNA libraries from Drosophila embryos. J Mol Biol. 1988;203:425–437. - PubMed
-
- Burton K, Taylor DL. Traction forces of cytokinesis measured with optically modified elastic substrata. Nature. 1997;385:450–454. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
