The ATP-dependent PIM1 protease is required for the expression of intron-containing genes in mitochondria
- PMID: 9585511
- PMCID: PMC316837
- DOI: 10.1101/gad.12.10.1515
The ATP-dependent PIM1 protease is required for the expression of intron-containing genes in mitochondria
Abstract
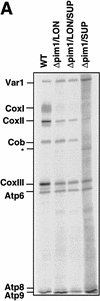
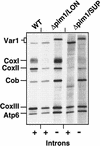
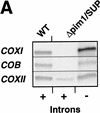
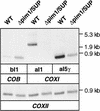
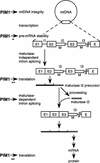
The ATP-dependent PIM1 protease, a Lon-like protease localized in the mitochondrial matrix, is required for mitochondrial genome integrity in yeast. Cells lacking PIM1 accumulate lesions in the mitochondrial DNA (mtDNA) and therefore lose respiratory competence. The identification of a multicopy suppressor, which stabilizes mtDNA in the absence of PIM1, enabled us to characterize novel functions of PIM1 protease during mitochondrial biogenesis. The synthesis of mitochondrially encoded cytochrome c oxidase subunit I (CoxI) and cytochrome b (Cob) is impaired in pim1 mutants containing mtDNA. PIM1-mediated proteolysis is required for the translation of mature COXI mRNA. Moreover, deficiencies in the splicing of COXI and COB transcripts, which appear to be restricted to introns encoding mRNA maturases, were observed in cells lacking the PIM1 gene. Transcripts of COXI and COB genes harboring multiple introns are degraded in the absence of PIM1. These results establish multiple, essential functions of the ATP-dependent PIM1 protease during mitochondrial gene expression.
Figures





Similar articles
-
The S. cerevisiae nuclear gene SUV3 encoding a putative RNA helicase is necessary for the stability of mitochondrial transcripts containing multiple introns.Curr Genet. 1995 Aug;28(3):217-24. doi: 10.1007/BF00309780. Curr Genet. 1995. PMID: 8529267
-
Substitution of PIM1 protease in mitochondria by Escherichia coli Lon protease.J Biol Chem. 1996 Apr 26;271(17):10137-42. doi: 10.1074/jbc.271.17.10137. J Biol Chem. 1996. PMID: 8626573
-
The MRS1 gene of S. douglasii: co-evolution of mitochondrial introns and specific splicing proteins encoded by nuclear genes.Gene Expr. 1992;2(3):203-14. Gene Expr. 1992. PMID: 1333316 Free PMC article.
-
ATP-dependent proteases controlling mitochondrial function in the yeast Saccharomyces cerevisiae.Cell Mol Life Sci. 1999 Nov 30;56(9-10):825-42. doi: 10.1007/s000180050029. Cell Mol Life Sci. 1999. PMID: 11212342 Free PMC article. Review.
-
RNA splicing in lower eukaryotes.Curr Opin Genet Dev. 1992 Oct;2(5):712-9. doi: 10.1016/s0959-437x(05)80131-5. Curr Opin Genet Dev. 1992. PMID: 1333856 Review.
Cited by
-
Loss of Lon1 in Arabidopsis changes the mitochondrial proteome leading to altered metabolite profiles and growth retardation without an accumulation of oxidative damage.Plant Physiol. 2012 Nov;160(3):1187-203. doi: 10.1104/pp.112.203711. Epub 2012 Sep 11. Plant Physiol. 2012. PMID: 22968828 Free PMC article.
-
The formation of respiratory chain complexes in mitochondria is under the proteolytic control of the m-AAA protease.EMBO J. 1998 Aug 17;17(16):4837-47. doi: 10.1093/emboj/17.16.4837. EMBO J. 1998. PMID: 9707443 Free PMC article.
-
Mitochondrial Lon protease at the crossroads of oxidative stress, ageing and cancer.Cell Mol Life Sci. 2015 Dec;72(24):4807-24. doi: 10.1007/s00018-015-2039-3. Epub 2015 Sep 12. Cell Mol Life Sci. 2015. PMID: 26363553 Free PMC article. Review.
-
Temperature-sensitive mutation in yeast mitochondrial ribosome recycling factor (RRF).Nucleic Acids Res. 2003 Jul 15;31(14):4218-26. doi: 10.1093/nar/gkg449. Nucleic Acids Res. 2003. PMID: 12853640 Free PMC article.
-
Obtusilactone A and (-)-sesamin induce apoptosis in human lung cancer cells by inhibiting mitochondrial Lon protease and activating DNA damage checkpoints.Cancer Sci. 2010 Dec;101(12):2612-20. doi: 10.1111/j.1349-7006.2010.01701.x. Cancer Sci. 2010. PMID: 21077998 Free PMC article.
References
-
- Adam Z. Protein stability and degradation in chloroplasts. Plant Mol Biol. 1996;32:773–783. - PubMed
-
- Arlt H, Tauer R, Feldmann H, Neupert W, Langer T. The YTA10-12-complex, an AAA protease with chaperone-like activity in the inner membrane of mitochondria. Cell. 1996;85:875–885. - PubMed
-
- Ausubel FJ, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K. Current protocols in molecular biology. New York, NY: Greene Publishing Associates and Wiley-Interscience; 1992.
-
- Baumeister W, Lupas A. The proteasome. Curr Opin Struct Biol. 1997;7:273–282. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
