Prevention of PC12 cell death by N-acetylcysteine requires activation of the Ras pathway
- PMID: 9592085
- PMCID: PMC6792807
- DOI: 10.1523/JNEUROSCI.18-11-04042.1998
Prevention of PC12 cell death by N-acetylcysteine requires activation of the Ras pathway
Abstract
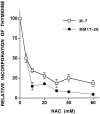
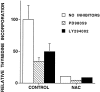
We have shown that N-acetylcysteine (NAC) promotes survival of sympathetic neurons and pheochromocytoma (PC12) cells in the absence of trophic factors. This action of NAC was not related to its antioxidant properties or ability to increase intracellular glutathione levels but was instead dependent on ongoing transcription and seemed attributable to the action of NAC as a reducing agent. Here, we investigate the mechanism by which NAC promotes neuronal survival. We show that NAC activates the Ras-extracellular signal-regulated kinase (ERK) pathway in PC12 cells. Ras activation by NAC seems necessary for survival in that it is unable to sustain serum-deprived PC12 MM17-26 cells constitutively expressing a dominant-negative form of Ras. Promotion of PC12 cell survival by NAC is totally blocked by PD98059, an inhibitor of the ERK-activating MAP kinase/ERK kinase, suggesting a required role for ERK activation in the NAC mechanism. In contrast, LY294002 and wortmannin, inhibitors of phosphatidylinositol 3-kinase (PI3K) that partially block NGF-promoted PC12 cell survival, have no effect on prevention of death by NAC. We hypothesized previously that the ability of NAC to promote survival correlates with its antiproliferative properties. However, although NAC does not protect PC12 MM17-26 cells from loss of trophic support, it does inhibit their capacity to synthesize DNA. Thus, the antiproliferative effect of NAC does not require Ras activation, and inhibition of DNA synthesis is insufficient to mediate NAC-promoted survival. These findings highlight the role of Ras-ERK activation in the mechanism by which NAC prevents neuronal death after loss of trophic support.
Figures







References
-
- Abate C, Patel L, Rauscher FJ, III, Curran T. Redox regulation of fos and jun DNA-binding activity in vitro. Science. 1990;249:1157–1161. - PubMed
-
- Alessi DR, Cuenda A, Cohen O, Dudley DT, Saltiel AR. PD98059 is a specific inhibitor of the activation of mitogen-activated protein kinases kinase in vitro and in vivo. J Biol Chem. 1995;270:27489–27494. - PubMed
-
- Aruoma OJ, Halliwell B, Hoey BM, Butler J. The antioxidant action of N-acetylcysteine: its reaction with hydrogen peroxide, hydroxyl radical, superoxide and hypochlorous acid. Free Radic Biol Med. 1989;6:593–597. - PubMed
-
- Bar-Sagi D, Feramisco JR. Microinjection of the ras oncogene protein into PC12 cells induces morphological differentiation. Cell. 1985;42:841–848. - PubMed
-
- Basu T, Warne PH, Downward J. Role of Shc in the activation of Ras in response to epidermal growth factor and nerve growth factor. Oncogene. 1994;9:3483–3491. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
