Modulation of rat rotational behavior by direct gene transfer of constitutively active protein kinase C into nigrostriatal neurons
- PMID: 9592092
- PMCID: PMC6792804
- DOI: 10.1523/JNEUROSCI.18-11-04119.1998
Modulation of rat rotational behavior by direct gene transfer of constitutively active protein kinase C into nigrostriatal neurons
Abstract
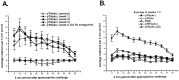
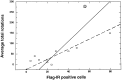
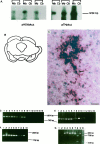
The modulation of motor behavior by protein kinase C (PKC) signaling pathways in nigrostriatal neurons was examined by using a genetic intervention approach. Herpes simplex virus type 1 (HSV-1) vectors that encode a catalytic domain of rat PKCbetaII (PkcDelta) were developed. PkcDelta exhibited a constitutively active protein kinase activity with a substrate specificity similar to that of rat brain PKC. As demonstrated in cultured sympathetic neurons, PkcDelta caused a long-lasting, activation-dependent increase in neurotransmitter release. In the rat brain, microinjection of HSV-1 vectors that contain the tyrosine hydroxylase promoter targeted expression to dopaminergic nigrostriatal neurons. Expression of pkcDelta in a small percentage of nigrostriatal neurons (approximately 0.1-2%) was sufficient to produce a long-term (>/=1 month) change in apomorphine-induced rotational behavior. Nigrostriatal neurons were the only catecholaminergic neurons that contained PkcDelta, and the amount of rotational behavior was correlated with the number of affected nigrostriatal neurons. The change in apomorphine-induced rotational behavior was blocked by a dopamine receptor antagonist (fluphenazine). D2-like dopamine receptor density was increased in those regions of the striatum innervated by the affected nigrostriatal neurons. Therefore, this strategy enabled the demonstration that a PKC pathway or PKC pathways in nigrostriatal neurons modulate apomorphine-induced rotational behavior, and altered dopaminergic transmission from nigrostriatal neurons appears to be the affected neuronal physiology responsible for the change in rotational behavior.
Figures


Similar articles
-
Genetic analysis of the role of protein kinase C signaling pathways in behaviors by direct gene transfer with HSV-1 vectors.Rev Neurosci. 1999;10(1):1-13. doi: 10.1515/revneuro.1999.10.1.1. Rev Neurosci. 1999. PMID: 10356988 Review.
-
Enhanced nigrostriatal neuron-specific, long-term expression by using neural-specific promoters in combination with targeted gene transfer by modified helper virus-free HSV-1 vector particles.BMC Neurosci. 2008 Apr 10;9:37. doi: 10.1186/1471-2202-9-37. BMC Neurosci. 2008. PMID: 18402684 Free PMC article.
-
Reformation of the nigrostriatal pathway by fetal dopaminergic micrografts into the substantia nigra is critically dependent on the age of the host.Exp Neurol. 1999 Sep;159(1):177-90. doi: 10.1006/exnr.1999.7110. Exp Neurol. 1999. PMID: 10486186
-
Targeted gene transfer to nigrostriatal neurons in the rat brain by helper virus-free HSV-1 vector particles that contain either a chimeric HSV-1 glycoprotein C-GDNF or a gC-BDNF protein.Brain Res Mol Brain Res. 2005 Sep 13;139(1):88-102. doi: 10.1016/j.molbrainres.2005.05.029. Brain Res Mol Brain Res. 2005. PMID: 15993510 Free PMC article.
-
Priming of a D1 dopamine receptor behavioural response is dissociated from striatal immediate-early gene activity.Neuroscience. 1995 May;66(2):347-59. doi: 10.1016/0306-4522(94)00582-p. Neuroscience. 1995. PMID: 7477877
Cited by
-
Antibody-mediated targeted gene transfer to NMDA NR1-containing neurons in rat neocortex by helper virus-free HSV-1 vector particles containing a chimeric HSV-1 glycoprotein C-staphylococcus A protein.Brain Res. 2010 Sep 10;1351:1-12. doi: 10.1016/j.brainres.2010.06.045. Epub 2010 Jun 28. Brain Res. 2010. PMID: 20599821 Free PMC article.
-
Regulation of phospholipase Cgamma in the mesolimbic dopamine system by chronic morphine administration.J Neurochem. 1999 Oct;73(4):1520-8. doi: 10.1046/j.1471-4159.1999.0731520.x. J Neurochem. 1999. PMID: 10501197 Free PMC article.
-
Efficient gene transfers into neocortical neurons connected by NMDA NR1-containing synapses.J Neurosci Methods. 2019 Nov 1;327:108390. doi: 10.1016/j.jneumeth.2019.108390. Epub 2019 Aug 9. J Neurosci Methods. 2019. PMID: 31404560 Free PMC article.
-
Separate Gene Transfers into Pre- and Postsynaptic Neocortical Neurons Connected by mGluR5-Containing Synapses.J Mol Neurosci. 2019 Aug;68(4):549-564. doi: 10.1007/s12031-019-01317-9. Epub 2019 Apr 10. J Mol Neurosci. 2019. PMID: 30972540 Free PMC article.
-
Herpes simplex virus type 1/adeno-associated virus rep(+) hybrid amplicon vector improves the stability of transgene expression in human cells by site-specific integration.J Virol. 2002 Jul;76(14):7150-62. doi: 10.1128/jvi.76.14.7150-7162.2002. J Virol. 2002. PMID: 12072515 Free PMC article.
References
-
- Abeliovich A, Chen C, Goda Y, Silva AJ, Stevens CF, Tonegawa S. Modified hippocampal long-term potentiation in PKCγ-mutant mice. Cell. 1993a;75:1253–1262. - PubMed
-
- Abeliovich A, Paylor R, Chen C, Kim JJ, Wehner JM, Tonegawa S. PKCγ mutant mice exhibit mild deficits in spatial and contextual learning. Cell. 1993b;75:1263–1271. - PubMed
-
- Akers RF, Routtenberg A. Protein kinase C phosphorylates a 47 Mr protein (F1) directly related to synaptic plasticity. Brain Res. 1985;334:147–151. - PubMed
-
- Arbuthnott GW, Ungerstedt U. Turning behavior induced by electrical stimulation of the nigro-striatal system of the rat. Exp Neurol. 1975;47:162–172. - PubMed
-
- Bruno JP, Stricker EM, Zigmond MJ. Rats given dopamine-depleting brain lesions as neonates are subsensitive to dopaminergic antagonists as adults. Behav Neurosci. 1985;99:771–775. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
