Induced gamma-band activity during the delay of a visual short-term memory task in humans
- PMID: 9592102
- PMCID: PMC6792803
- DOI: 10.1523/JNEUROSCI.18-11-04244.1998
Induced gamma-band activity during the delay of a visual short-term memory task in humans
Abstract
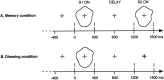
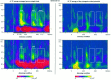
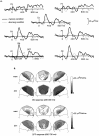
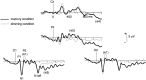
It has been hypothesized that visual objects could be represented in the brain by a distributed cell assembly synchronized on an oscillatory mode in the gamma-band (20-80 Hz). If this hypothesis is correct, then oscillatory gamma-band activity should appear in any task requiring the activation of an object representation, and in particular when an object representation is held active in short-term memory: sustained gamma-band activity is thus expected during the delay of a delayed-matching-to-sample task. EEG was recorded while subjects performed such a task. Induced (e.g., appearing with a jitter in latency from one trial to the next) gamma-band activity was observed during the delay. In a control task, in which no memorization was required, this activity disappeared. Furthermore, this gamma-band activity during the rehearsal of the first stimulus representation in short-term memory peaked at both occipitotemporal and frontal electrodes. This topography fits with the idea of a synchronized cortical network centered on prefrontal and ventral visual areas. Activities in the alpha band, in the 15-20 Hz band, and in the averaged evoked potential were also analyzed. The gamma-band activity during the delay can be distinguished from all of these other components of the response, on the basis of either its variations or its topography. It thus seems to be a specific functional component of the response that could correspond to the rehearsal of an object representation in short-term memory.
Figures
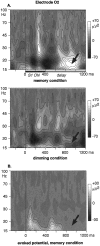
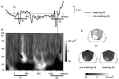

References
-
- Anllo-Vento L, Hillyard SA. Selective attention to the color and direction of moving stimuli: electrophysiological correlates of hierarchical feature selection. Percept Psychophys. 1996;58:191–206. - PubMed
-
- Begleiter H, Porjesz B, Wang W. A neurophysiologic correlate of visual short-term memory in humans. Electroenceph Clin Neurophysiol. 1993;87:46–53. - PubMed
-
- Brosch M, Bauer R, Eckhorn R. Stimulus-dependent modulations of correlated high-frequency oscillations in cat visual cortex. Cereb Cortex. 1997;7:70–76. - PubMed
-
- Courtney SM, Ungerleider LG, Keil K, Haxby JV. Object and spatial visual working memory activate separate neural systems in human cortex. Cereb Cortex. 1996;6:39–49. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
