Biological characterization of endotoxins released from antibiotic-treated Pseudomonas aeruginosa and Escherichia coli
- PMID: 9593119
- PMCID: PMC105737
- DOI: 10.1128/AAC.42.5.1015
Biological characterization of endotoxins released from antibiotic-treated Pseudomonas aeruginosa and Escherichia coli
Abstract
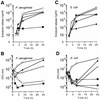
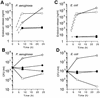
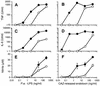
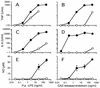
The supernatants taken from Pseudomonas aeruginosa and Escherichia coli cultures in human sera or chemically defined M9 medium in the presence of ceftazidime (CAZ) contained high levels of endotoxin, while those taken from the same cultures in the presence of imipenem (IPM) yielded a very low level of endotoxin. The biological activities of endotoxin in the supernatants were compared with those of phenol water-extracted lipopolysaccharide (LPS). The endotoxin released from the organisms as a result of CAZ treatment (CAZ-released endotoxin) contained a large amount of protein. The protein, however, lacked endotoxic activity, since the endotoxin did not show any in vivo toxic effects in LPS-hyporesponsive C3H/HeJ mice sensitized with D-(+)-galactosamine (GalN) or any activation of C3H/HeJ mouse macrophages in vitro. The activities of CAZ- and IPM-released endotoxin (as assessed by a chromogenic Limulus test) were fundamentally the same as those of P. aeruginosa LPS, since their regression lines were parallel. The CAZ-released endotoxin was similar to purified LPS with respect to the following biological activities in LPS-responsive C3H/HeN mice and LPS-hyporesponsive C3H/HeJ mice: lethal toxicity in GalN-sensitized mice, in vitro induction of tumor necrosis factor- and NO production by macrophages, and mitogen-activated protein kinase activation in macrophages. The macrophage activation by CAZ-released endotoxin as well as LPS was mainly dependent on the presence of serum factor and CD14 antigen. Polymyxin B blocked the activity. These findings indicate that the endotoxic activity of CAZ-released endotoxin is due primarily to LPS (lipid A).
Figures



Similar articles
-
Clindamycin suppresses endotoxin released by ceftazidime-treated Escherichia coli O55:B5 and subsequent production of tumor necrosis factor alpha and interleukin-1 beta.Antimicrob Agents Chemother. 1999 Mar;43(3):616-22. doi: 10.1128/AAC.43.3.616. Antimicrob Agents Chemother. 1999. PMID: 10049276 Free PMC article.
-
[Effects of isepamicin and beta-lactam antibiotics on the release of endotoxin from Pseudomonas aeruginosa].Jpn J Antibiot. 1999 Jan;52(1):41-56. Jpn J Antibiot. 1999. PMID: 10202687 Japanese.
-
Biological activities of lipopolysaccharides of Proteus spp. and their interactions with polymyxin B and an 18-kDa cationic antimicrobial protein (CAP18)-derived peptide.J Med Microbiol. 2000 Feb;49(2):127-138. doi: 10.1099/0022-1317-49-2-127. J Med Microbiol. 2000. PMID: 10670563
-
Mechanisms of endotoxin shock and endotoxin hypersensitivity.Immunobiology. 1993 Apr;187(3-5):346-56. doi: 10.1016/S0171-2985(11)80349-9. Immunobiology. 1993. PMID: 8330903 Review.
-
Interaction of nanoparticles with endotoxin Importance in nanosafety testing and exploitation for endotoxin binding.Nanotoxicology. 2021 May;15(4):558-576. doi: 10.1080/17435390.2021.1898690. Epub 2021 Mar 30. Nanotoxicology. 2021. PMID: 33784953 Review.
Cited by
-
The Anti-Microbial Peptide (Lin-SB056-1)2-K Reduces Pro-Inflammatory Cytokine Release through Interaction with Pseudomonas aeruginosa Lipopolysaccharide.Antibiotics (Basel). 2020 Sep 8;9(9):585. doi: 10.3390/antibiotics9090585. Antibiotics (Basel). 2020. PMID: 32911618 Free PMC article.
-
Interactions among strategies associated with bacterial infection: pathogenicity, epidemicity, and antibiotic resistance.Clin Microbiol Rev. 2002 Oct;15(4):647-79. doi: 10.1128/CMR.15.4.647-679.2002. Clin Microbiol Rev. 2002. PMID: 12364374 Free PMC article. Review.
-
Nitrite reductase from Pseudomonas aeruginosa released by antimicrobial agents and complement induces interleukin-8 production in bronchial epithelial cells.Antimicrob Agents Chemother. 1999 Apr;43(4):794-801. doi: 10.1128/AAC.43.4.794. Antimicrob Agents Chemother. 1999. PMID: 10103183 Free PMC article.
-
Modulating Laying Hens Productivity and Immune Performance in Response to Oxidative Stress Induced by E. coli Challenge Using Dietary Propolis Supplementation.Antioxidants (Basel). 2020 Sep 21;9(9):893. doi: 10.3390/antiox9090893. Antioxidants (Basel). 2020. PMID: 32967097 Free PMC article.
-
Pretreatment of mice with clindamycin improves survival of endotoxic shock by modulating the release of inflammatory cytokines.Antimicrob Agents Chemother. 2001 Sep;45(9):2638-42. doi: 10.1128/AAC.45.9.2638-2642.2001. Antimicrob Agents Chemother. 2001. PMID: 11502543 Free PMC article.
References
-
- Arditi M, Zhou J, Torres M, Durden D L, Stins M, Kim K S. Lipopolysaccharide stimulates the tyrosine phosphorylation of mitogen-activated protein kinases p44, p42, and p41 in vascular endothelial cells in a soluble CD14-dependent manner. Role of protein tyrosine phosphorylation in lipopolysaccharide-induced stimulation of endothelial cells. J Immunol. 1995;155:3994–4003. - PubMed
-
- Atlas R M. M9 medium. In: Parks L C, editor. Handbook of microbiological media. Vol. 529. Boca Raton, Fla: CRC Press, Inc.; 1993.
-
- Beaty C D, Franklin T L, Uehara Y, Wilson C B. Lipopolysaccharide induced cytokine production in human monocytes: role of tyrosine phosphorylation in transmembrane signal transduction. Eur J Immunol. 1994;24:1278–1284. - PubMed
-
- Bucklin S E, Morrison D C. Differences in therapeutic efficacy among cell wall-active antibiotics in a mouse model of gram-negative sepsis. J Infect Dis. 1995;172:1519–1527. - PubMed
-
- Chen T-Y, Bright S W, Pace J L, Russell S W, Morrison D C. Induction of macrophage-mediated tumor cytotoxicity by a hamster monoclonal antibody with specificity for a lipopolysaccharide receptor. J Immunol. 1990;145:8–12. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

