Metabolism in human cells of the D and L enantiomers of the carbocyclic analog of 2'-deoxyguanosine: substrate activity with deoxycytidine kinase, mitochondrial deoxyguanosine kinase, and 5'-nucleotidase
- PMID: 9593124
- PMCID: PMC105742
- DOI: 10.1128/AAC.42.5.1045
Metabolism in human cells of the D and L enantiomers of the carbocyclic analog of 2'-deoxyguanosine: substrate activity with deoxycytidine kinase, mitochondrial deoxyguanosine kinase, and 5'-nucleotidase
Abstract
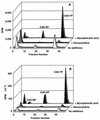
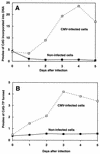
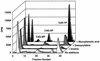
The carbocyclic analog of 2'-deoxyguanosine (CdG) has broad-spectrum antiviral activity. Because of recent observations with other nucleoside analogs that biological activity may be associated the L enantiomer rather than, as expected, with the D enantiomer, we have studied the metabolism of both enantiomers of CdG to identify the enzymes responsible for the phosphorylation of CdG in noninfected and virally infected human and duck cells. We have examined the enantiomers as substrates for each of the cellular enzymes known to catalyze phosphorylation of deoxyguanosine. Both enantiomers of CdG were substrates for deoxycytidine kinase (EC 2.7.1.74) from MOLT-4 cells, 5'-nucleotidase (EC 3.1.3.5) from HEp-2 cells, and mitochondrial deoxyguanosine kinase (EC 2.7.1.113) from human platelets and CEM cells. For both deoxycytidine kinase and mitochondrial deoxyguanosine kinase, the L enantiomer was the better substrate. Even though the D enantiomer was the preferred substrate with 5'-nucleotidase, the rate of phosphorylation of the L enantiomer was substantial. The phosphorylation of D-CdG in MRC-5 cells was greatly stimulated by infection with human cytomegalovirus. The fact that the phosphorylation of D-CdG was stimulated by mycophenolic acid and was not affected by deoxycytidine suggested that 5'-nucleotidase was the enzyme primarily responsible for its metabolism in virally infected cells. D-CdG was extensively phosphorylated in duck hepatocytes, and its phosphorylation was not affected by infection with duck hepatitis B virus. These results are of importance in understanding the mode of action of D-CdG and related analogs and in the design of new biologically active analogs.
Figures

References
-
- Allan P W, Parker W B, Arnett G, Rose L M, Shaddix S C, Shewach D S, Fourel I, Secrist III J A, Montgomery J A, Shealy Y F, Bennett L L., Jr Metabolism of the carbocyclic analog of 2′-deoxyguanosine (CdG) in human cells. Antivir Res. 1995;26:A266.
-
- Arner E S J, Eriksson S. Mammalian deoxyribonucleoside kinases. Pharmacol Ther. 1995;67:155–186. - PubMed
-
- Balzarini J, De Clercq E, Baumgartner H, Bodenteich M, Griengl H. Carbocyclic 5-iodo-2′-deoxyuridine (C-IDU) and carbocyclic (E)-5-(2-bromovinyl)-2′-deoxyuridine (C-BVDU) as unique examples of chiral molecules where the two enantiomeric forms are biologically active: interaction of the (+)- and (−)-enantiomers of C-IDU and C-BVDU with the thymidine kinase of herpes simplex virus type 1. Mol Pharmacol. 1990;37:395–401. - PubMed
-
- Bennett L L, Jr, Shealy Y F, Allan P W, Rose L M, Shannon W M, Arnett G. Phosphorylation of the carbocyclic analog of 2′-deoxyguanosine in cells infected with herpes viruses. Biochem Pharmacol. 1990;40:1515–1522. - PubMed
-
- Bennett L L, Jr, Parker W B, Allan P W, Rose L M, Shealy Y F, Secrist III J A, Montgomery J A, Arnett G, Kirkman R L, Shannon W M. Phosphorylation of the enantiomers of the carbocyclic analog of 2′-deoxyguanosine in cells infected with herpes simplex virus type 1 and in uninfected cells. Lack of enantiomeric selectivity with the viral thymidine kinase. Mol Pharmacol. 1993;44:1258–1266. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

