Sorivudine versus acyclovir for treatment of dermatomal herpes zoster in human immunodeficiency virus-infected patients: results from a randomized, controlled clinical trial. Collaborative Antiviral Study Group/AIDS Clinical Trials Group, Herpes Zoster Study Group
- PMID: 9593141
- PMCID: PMC105759
- DOI: 10.1128/AAC.42.5.1139
Sorivudine versus acyclovir for treatment of dermatomal herpes zoster in human immunodeficiency virus-infected patients: results from a randomized, controlled clinical trial. Collaborative Antiviral Study Group/AIDS Clinical Trials Group, Herpes Zoster Study Group
Abstract
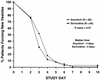
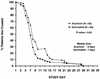
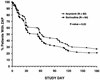
The present randomized, double-blind, placebo-controlled, multicenter clinical trial was designed to compare the efficacy and tolerability of sorivudine [1-beta-D-arabinofuranosyl-E-(2-bromovinyl)uracil] and acyclovir for the treatment of dermatomal herpes zoster in human immunodeficiency virus (HIV)-seropositive patients. A total of 170 HIV-seropositive adults presenting with herpes zoster (confirmed by direct fluorescent-antigen testing and/or viral culture) were enrolled and randomized to receive a 10-day course of orally administered sorivudine (40 mg once daily plus acyclovir placebos) or acyclovir (800 mg five times daily plus sorivudine placebo). Patients were monitored daily to document the events of cutaneous healing, pain, zoster-related complications, and drug-related adverse events. Patients were reassessed on days 21 and 28 and then once monthly for 1 year. The primary efficacy endpoint was time to the cessation of new vesicle formation. Secondary efficacy endpoints included times to other events of cutaneous healing, resolution of pain, and frequency of dissemination and zoster recurrence. In a multivariate analysis, sorivudine was superior to acyclovir for reducing the times to the cessation of new vesicle formation (relative risk [RR] = 1.54, 95% confidence interval [CI] = 1.00 to 2.36; P = 0.049) and total lesion crusting (RR = 1.48, 95% CI = 1.07 to 2.04; P = 0.017). In a univariate analysis, there was a trend favoring sorivudine for the cessation of new vesicle formation (median of 3 versus 4 days; P = 0.07) and a significant advantage for time to total lesion crusting (median of 7 versus 8 days; P = 0.02). The time to the resolution of zoster-associated pain, the frequency of dissemination, and the frequency of zoster recurrence were not different between the two treatment groups. Both drugs were well tolerated. Sorivudine is an effective drug for the treatment of herpes zoster in HIV-infected patients and results in accelerated cutaneous healing when compared with acyclovir therapy.
Figures
References
-
- Balfour H H, Bean B, Laskin O L, Ambinder R F, Meyers J D, Wade J C, Zaia J A, Aeppli D, Kirk L E, Segreti A C, Keeney R E Burroughs Wellcome Collaborative Antiviral Study Group. Acyclovir halts progression of herpes zoster in immunocompromised patients. N Engl J Med. 1983;308:1448–1453. - PubMed
-
- Bodsworth N J, Boag F, Burdge D, Généreux M, Borleffs J C C, Evans B A, Modai J, Colebunders R, Thomis J the Multinational Sorivudine Study Group. Evaluation of sorivudine (BV-araU) versus acyclovir in the treatment of acute localized herpes zoster in human immunodeficiency virus-infected adults. J Infect Dis. 1997;176:103–111. - PubMed
-
- Buchbinder S P, Kate M H, Hessol N A, Lin J Y, O’Malley P M, Underwood R, Holberg S D. Herpes zoster and human immunodeficiency virus infection. J Infect Dis. 1992;166:1153–1156. - PubMed
-
- Desgranges C, Razaka G, DeClerq E, Herdewijn P, Balzarini J, Drouillet F, Bricaud H. Effect of E-5-(2-bromovinyl)uracil on the catabolism and antitumor activity of 5-fluorouracil in rats and leukemic mice. Cancer Res. 1986;46:1094–1101. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

