Defluorinated sparfloxacin as a new photoproduct identified by liquid chromatography coupled with UV detection and tandem mass spectrometry
- PMID: 9593143
- PMCID: PMC105763
- DOI: 10.1128/AAC.42.5.1151
Defluorinated sparfloxacin as a new photoproduct identified by liquid chromatography coupled with UV detection and tandem mass spectrometry
Abstract
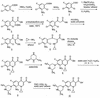
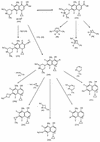
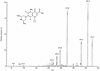
Photodegradation of sparfloxacin was observed by means of high-pressure liquid chromatography with UV detection and liquid chromatography coupled with UV detection and tandem mass spectrometry (LC-MS/MS). Three products were detected. Comparison with an independently synthesized derivative of sparfloxacin revealed the structure of one product which is believed to be 8-desfluorosparfloxacin. The second product is likely to be formed by the splitting off of a fluorine and a cyclopropyl ring. Thus, photodefluorination of quinolone antibacterial agents is found and proved for the first time by LC-MS/MS.
Figures






Similar articles
-
Characterization of clinafloxacin photodegradation products by LC-MS/MS and NMR.J Pharm Biomed Anal. 2000 Aug 15;23(2-3):521-34. doi: 10.1016/s0731-7085(00)00330-7. J Pharm Biomed Anal. 2000. PMID: 10933546
-
Comparative uptake of sparfloxacin and ciprofloxacin into human THP 1 monocytic cells.Arzneimittelforschung. 1996 Mar;46(3):316-9. Arzneimittelforschung. 1996. PMID: 8901157
-
Photodegradation of some quinolones used as antimicrobial therapeutics.J Pharm Sci. 1994 Apr;83(4):463-7. doi: 10.1002/jps.2600830403. J Pharm Sci. 1994. PMID: 8046597
-
Sparfloxacin worldwide in vitro literature: isolate data available through 1994.Diagn Microbiol Infect Dis. 1996 Jun;25(2):53-64. doi: 10.1016/s0732-8893(96)00121-6. Diagn Microbiol Infect Dis. 1996. PMID: 8882890 Review.
-
Determination of aminoglycosides and quinolones in food using tandem mass spectrometry: a review.Crit Rev Food Sci Nutr. 2004;44(3):173-84. doi: 10.1080/10408690490441488. Crit Rev Food Sci Nutr. 2004. PMID: 15239371 Review.
Cited by
-
Microbial transformations of antimicrobial quinolones and related drugs.J Ind Microbiol Biotechnol. 2012 Dec;39(12):1731-40. doi: 10.1007/s10295-012-1194-x. Epub 2012 Sep 25. J Ind Microbiol Biotechnol. 2012. PMID: 23007957 Review.
-
Evaluation of sparfloxacin distribution by mass spectrometry imaging in a phototoxicity model.J Am Soc Mass Spectrom. 2014 Oct;25(10):1803-9. doi: 10.1007/s13361-014-0947-3. Epub 2014 Jul 8. J Am Soc Mass Spectrom. 2014. PMID: 25001383
-
Modification of norfloxacin by a Microbacterium sp. strain isolated from a wastewater treatment plant.Appl Environ Microbiol. 2011 Sep;77(17):6100-8. doi: 10.1128/AEM.00545-11. Epub 2011 Jul 1. Appl Environ Microbiol. 2011. PMID: 21724893 Free PMC article.
-
Current advances on the photocatalytic degradation of fluoroquinolones: photoreaction mechanism and environmental application.Photochem Photobiol Sci. 2022 May;21(5):899-912. doi: 10.1007/s43630-022-00217-z. Epub 2022 Apr 13. Photochem Photobiol Sci. 2022. PMID: 35416639 Review.
References
-
- Detzer N, Huber B. Photochemie heterocyclischer Enone I, Photolyse und Thermolyse von Nalidixinsäure. Tetrahedron. 1975;31:1937–1941.
-
- Engler M, Holzgrabe U, Kinzig M, Sörgel F. Abstracts of papers of the American Chemical Society. Vol. 211. 1996. Photoproducts of sparfloxacin identified by means of LC-MS/MS; pp. 173–medi. , pt. 2.
-
- Jürgens J, Schedletzky H, Heisig P, Seydel J K, Wiedemann B, Holzgrabe U. Syntheses and biological activities of new N1-aryl substituted quinolone antibacterials. Arch Pharm Med Chem. 1996;329:179–190. - PubMed
-
- Martinez L J, Li G, Chignell C F. Photogeneration of fluoride by the fluoroquinolone antimicrobial agents lomefloxacin and fleroxacin. Photochem Photobiol. 1997;65:599–602. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

