Steady-state pharmacokinetics and electrocardiographic pharmacodynamics of clarithromycin and loratadine after individual or concomitant administration
- PMID: 9593146
- PMCID: PMC105769
- DOI: 10.1128/AAC.42.5.1176
Steady-state pharmacokinetics and electrocardiographic pharmacodynamics of clarithromycin and loratadine after individual or concomitant administration
Abstract
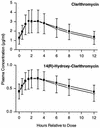
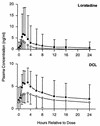
To evaluate the potential for an interaction between clarithromycin and loratadine, healthy male volunteers (n = 24) received each of the following regimens according to a randomized crossover design: 500 mg of clarithromycin orally every 12 h (q12h) for 10 days, 10 mg of loratadine orally q24h for 10 days, and the combination of clarithromycin and loratadine. A washout interval of 14 days separated regimens. The addition of loratadine did not statistically significantly affect the steady-state pharmacokinetics of clarithromycin or its active metabolite, 14(R)-hydroxy-clarithromycin. However, the addition of clarithromycin statistically significantly altered the steady-state maximum observed plasma concentration and the area under the plasma concentration-time curve over a dosing interval for loratadine (+36 and +76%, respectively) and for descarboethoxyloratadine (DCL), the active metabolite of loratadine (+69 and +49%, respectively). Clarithromycin probably inhibits the oxidative metabolism of loratadine and DCL by the cytochrome P-450 3A subfamily. Electrocardiograms (n = 12) were obtained over 24-h periods at baseline and steady state (day 10). The mean maximum QTc interval and area under the QTc interval-time curve on day 10 were modestly increased (<3%) from baseline for all three regimens, but no QTc interval exceeded 439 ms for any subject. Elevated steady-state concentrations of loratadine and DCL do not appear to be associated with adverse cardiovascular effects related to prolongation of the QTc interval. Loratadine and clarithromycin were well tolerated, alone and in combination.
Figures
Similar articles
-
Loratadine administered concomitantly with erythromycin: pharmacokinetic and electrocardiographic evaluations.Clin Pharmacol Ther. 1995 Sep;58(3):269-78. doi: 10.1016/0009-9236(95)90243-0. Clin Pharmacol Ther. 1995. PMID: 7554700 Clinical Trial.
-
Evaluation of the pharmacokinetics and electrocardiographic pharmacodynamics of loratadine with concomitant administration of ketoconazole or cimetidine.Br J Clin Pharmacol. 2000 Dec;50(6):581-9. doi: 10.1046/j.1365-2125.2000.00290.x. Br J Clin Pharmacol. 2000. PMID: 11136297 Free PMC article. Clinical Trial.
-
Loratadine and terfenadine interaction with nefazodone: Both antihistamines are associated with QTc prolongation.Clin Pharmacol Ther. 2001 Mar;69(3):96-103. doi: 10.1067/mcp.2001.114230. Clin Pharmacol Ther. 2001. PMID: 11240972 Clinical Trial.
-
Clinical pharmacokinetics of clarithromycin.Clin Pharmacokinet. 1999 Nov;37(5):385-98. doi: 10.2165/00003088-199937050-00003. Clin Pharmacokinet. 1999. PMID: 10589373 Review.
-
Clarithromycin clinical pharmacokinetics.Clin Pharmacokinet. 1993 Sep;25(3):189-204. doi: 10.2165/00003088-199325030-00003. Clin Pharmacokinet. 1993. PMID: 8222460 Review.
Cited by
-
Clinical pharmacology of new histamine H1 receptor antagonists.Clin Pharmacokinet. 1999 May;36(5):329-52. doi: 10.2165/00003088-199936050-00003. Clin Pharmacokinet. 1999. PMID: 10384858 Review.
-
Macrolide - induced clinically relevant drug interactions with cytochrome P-450A (CYP) 3A4: an update focused on clarithromycin, azithromycin and dirithromycin.Br J Clin Pharmacol. 2000 Oct;50(4):285-95. doi: 10.1046/j.1365-2125.2000.00261.x. Br J Clin Pharmacol. 2000. PMID: 11012550 Free PMC article. Review. No abstract available.
-
Improving antibacterial prescribing safety in the management of COPD exacerbations: systematic review of observational and clinical studies on potential drug interactions associated with frequently prescribed antibacterials among COPD patients.J Antimicrob Chemother. 2019 Oct 1;74(10):2848-2864. doi: 10.1093/jac/dkz221. J Antimicrob Chemother. 2019. PMID: 31127283 Free PMC article.
-
Drug-drug interactions and QT prolongation as a commonly assessed cardiac effect - comprehensive overview of clinical trials.BMC Pharmacol Toxicol. 2016 Mar 10;17:12. doi: 10.1186/s40360-016-0053-1. BMC Pharmacol Toxicol. 2016. PMID: 26960809 Free PMC article. Review.
-
A human model of Buruli ulcer: Provisional protocol for a Mycobacterium ulcerans controlled human infection study.Wellcome Open Res. 2024 Oct 21;9:488. doi: 10.12688/wellcomeopenres.22719.2. eCollection 2024. Wellcome Open Res. 2024. PMID: 39386965 Free PMC article.
References
-
- Affrime M B, Lorber R, Danzig M, Cuss F, Brannan M D. Three month evaluation of electrocardiographic effects of loratadine in humans. J Allergy Clin Immunol. 1993;91:259.
-
- Benedict C R. The QT interval and drug-associated torsades de pointes. Drug Invest. 1993;5:69–79.
-
- Brannan M D, Reidenberg P, Radwanski E, Shneyer L, Lin C C, Cayen M N, Affrime M B. Loratadine administered concomitantly with erythromycin: pharmacokinetic and electrocardiographic evaluations. Clin Pharmacol Ther. 1995;58:269–278. - PubMed
-
- Chu S Y, Sennello L T, Sonders R C. Simultaneous determination of clarithromycin and 14(R)-hydroxyclarithromycin in plasma and urine using high-performance liquid chromatography with electrochemical detection. J Chromatogr. 1991;571:199–208. - PubMed
-
- Chu S Y, Wilson D S, Deaton R L, Mackenthun A V, Eason C N, Cavanaugh J H. Single- and multiple-dose pharmacokinetics of clarithromycin, a new macrolide antimicrobial. J Clin Pharmacol. 1993;33:719–726. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

