Novel inhibitory effects of gamma-glutamylcysteine ethyl ester against human immunodeficiency virus type 1 production and propagation
- PMID: 9593150
- PMCID: PMC105777
- DOI: 10.1128/AAC.42.5.1200
Novel inhibitory effects of gamma-glutamylcysteine ethyl ester against human immunodeficiency virus type 1 production and propagation
Abstract
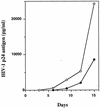
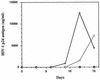
The anti-human immunodeficiency virus type I (anti-HIV-1) effects of gamma-glutamylcysteine ethyl ester (gamma-GCE; TEI-2306) were examined in vitro. In initial studies using a vigorously HIV-1-producing human T-lymphocytic cell line, gamma-GCE displayed a novel biphasic repressive effect on chronic HIV-1 infection that was unlike that of other glutathione prodrugs or other reported antioxidants. In high doses, up to a concentration of 2.5 mM, at which neither glutathione (GSH) nor another GSH precursor has shown inhibitory effects, gamma-GCE potently inhibited the production of HIV-1 by a selective cytopathic effect against infected cells, while the viability and growth of uninfected cells were unaffected at the same gamma-GCE concentrations. At lower concentrations (200 to 400 microM), gamma-GCE significantly repressed the virus production from chronically HIV-1-expressing cells without affecting their viability. The discrepancy of the thresholds of the toxic doses between infected and uninfected cells was found to be more than 10-fold. Relatively high doses of gamma-GCE, utilized in acute HIV-1 infection of T-lymphocytic cells, entirely blocked the propagation of HIV-1 and rescued the cells from HIV-1-induced cell death. Furthermore, gamma-GCE at such concentrations was found to directly inhibit the infectivity of HIV-1 within 4 h. Repressive effects of gamma-GCE on acute HIV-1 infection in human primary human peripheral blood mononuclear cells were also demonstrated. Here, the anti-HIV-1 strategy utilizing gamma-GCE is removal of both HIV-1-producing cells and free infectious HIV-1 in vitro, in place of specific immunoclearance in vivo, which might lead to an arrest or slowing of viral propagation in HIV-1-infected individuals.
Figures






References
-
- Aukrust P, Svardal A M, Muller F, Lunden B, Berge R K, Ueland P M, Froland S S. Increased levels of oxidized glutathione in CD4+ lymphocytes associated with disturbed intracellular redox balance in human immunodeficiency virus type 1 infection. Blood. 1995;86:258–267. - PubMed
-
- Buhl R, Jaffe H A, Holroyd K J, Wells F B, Mastrangeli A, Saltini C, Cantin A M, Crystal R G. Systemic glutathione deficiency in symptom-free HIV-seropositive individuals. Lancet. 1989;ii:1294–1298. - PubMed
-
- Cayota A, Vuillier F, Gonzalez G, Dighiero G. In vitro antioxidant treatment recovers proliferative responses of anergic CD4+ lymphocytes from human immunodeficiency virus-infected individuals. Blood. 1996;87:4746–4753. - PubMed
-
- Chandra A, Demirhan I, Arya S K, Chandra P. d-Penicillamine inhibits transactivation of human immunodeficiency virus type I (HIV-1) LTR by transactivator protein. FEBS Lett. 1988;236:282–286. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

