Risk of development of in vitro resistance to amoxicillin, clarithromycin, and metronidazole in Helicobacter pylori
- PMID: 9593154
- PMCID: PMC105783
- DOI: 10.1128/AAC.42.5.1222
Risk of development of in vitro resistance to amoxicillin, clarithromycin, and metronidazole in Helicobacter pylori
Abstract
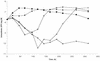
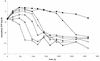
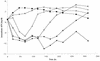
We have studied initial killing, morphological alterations, the frequency of occurrence, and the selective growth of resistant subpopulations of Helicobacter pylori during exposure to amoxicillin, clarithromycin, or metronidazole by bioluminescence assay of intracellular ATP levels, microscopy, and a viable count assay. We found an induction of spheroplasts and a decrease in intracellular ATP levels after 21 h of exposure to high concentrations of amoxicillin. During clarithromycin exposure the onset of a decrease in intracellular ATP levels started after prolonged incubation, and with the highest concentration of clarithromycin an induction of coccoid forms was seen after 68 h. Metronidazole exposure resulted in the strongest initial decrease in intracellular ATP levels, and coccoid forms were seen after 21 h of exposure to high concentrations of metronidazole. Amoxicillin caused a low-level increase in resistant subpopulations, which indicates a need for surveillance of the amoxicillin susceptibility of H. pylori in order to detect decreasing susceptibility. No increase in the numbers of resistant subpopulations was demonstrated during clarithromycin exposure. Metronidazole selected resistant subpopulations, which caused high-level resistance in H. pylori.
Figures



References
-
- Armstrong J A, Wee S H, Goodwin C S, Wilson D H. Response of Campylobacter pyloris to antibiotics, bismuth and an acid-reducing agent—an ultrastructural study. J Med Microbiol. 1987;24:343–350. - PubMed
-
- Bazzoli F, Zagari R M, Fossi S, Pozzato P, Alampi G, Simoni P, Sottili S, Roda A, Roda E. Short-term low-dose triple therapy for the eradication of Helicobacter pylori. Eur J Gastroenterol Hepatol. 1994;6:773–777.
-
- Bell G D, Powell K, Burridge S M, Bowden A F, Atoyebi W, Bolton G H, Jones P H, Browns C. Rapid eradication of Helicobacter pylori infection. Aliment Pharmacol Ther. 1995;9:41–46. - PubMed
-
- Bell G D, Powell K, Burridge S M, Pallecaros A, Jones P H, Gant P W, Harrison G, Trowell J E. Experience with “triple” anti-Helicobacter pylori eradication therapy: side effects and the importance of testing the pre-treatment bacterial isolate for metronidazole resistance. Aliment Pharmacol Ther. 1992;6:427–435. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

