Characterization of mannose receptor-dependent phagocytosis mediated by Mycobacterium tuberculosis lipoarabinomannan
- PMID: 9596746
- PMCID: PMC108268
- DOI: 10.1128/IAI.66.6.2769-2777.1998
Characterization of mannose receptor-dependent phagocytosis mediated by Mycobacterium tuberculosis lipoarabinomannan
Abstract
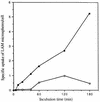
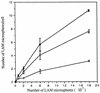
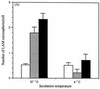
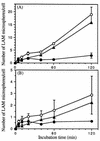
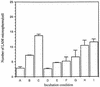
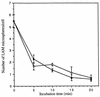
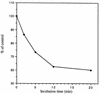
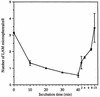
The macrophage mannose receptor (MR) along with complement receptors mediates phagocytosis of the M. tuberculosis virulent strains Erdman and H37Rv. We have determined that the terminal mannosyl units of the M. tuberculosis surface lipoglycan, lipoarabinomannan (LAM), from the Erdman strain serve as ligands for the MR. The biology of the MR (receptor binding and trafficking) in response to phagocytic stimuli is not well characterized. This study analyzes the MR-dependent phagocytosis mediated by Erdman LAM presented on a 1-micron-diameter phagocytic particle. Erdman LAM microspheres exhibited a time- and dose-dependent rapid increase in attachment and internalization by human monocyte-derived macrophages (MDMs). In contrast, internalization of LAM microspheres by monocytes was minimal. Microsphere internalization by MDMs was visualized and quantitated by immunofluorescence and confocal and electron microscopy and resembled conventional phagocytosis. Phagocytosis of LAM microspheres by MDMs was energy, cytoskeleton, and calcium dependent and was mannan inhibitable. Trypsin treatment of MDMs at 37 degrees C, which depleted surface and recycling intracellular pools of the MR, reduced the subsequent attachment of LAM microspheres. Trypsin treatment at 4 degrees C allowed for subsequent recovery of LAM microsphere phagocytosis at 37 degrees C by recycled MRs. Pretreatment of MDMs with cycloheximide influenced LAM microsphere phagocytosis to only a small extent, indicating that MR-dependent phagocytosis of the microspheres was occurring primarily by preformed recycled receptors. This study characterizes the requirements for macrophage phagocytosis of a LAM-coated particle mediated by the MR. This model will be useful in further characterization of the intracellular pathway taken by phagocytic particles coated with different LAM types in macrophages following ingestion.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

