Characterization of protective epitopes in a highly conserved Plasmodium falciparum antigenic protein containing repeats of acidic and basic residues
- PMID: 9596765
- PMCID: PMC108287
- DOI: 10.1128/IAI.66.6.2895-2904.1998
Characterization of protective epitopes in a highly conserved Plasmodium falciparum antigenic protein containing repeats of acidic and basic residues
Abstract
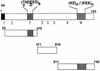
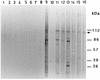
The delineation of putatively protective and immunogenic epitopes in vaccine candidate proteins constitutes a major research effort towards the development of an effective malaria vaccine. By virtue of its role in the formation of the immune clusters of merozoites, its location on the surface of merozoites, and its highly conserved nature both at the nucleotide sequence level and the amino acid sequence level, the antigen which contains repeats of acidic and basic residues (ABRA) of the human malaria parasite Plasmodium falciparum represents such an antigen. Based upon the predicted amino acid sequence of ABRA, we synthesized eight peptides, with six of these (AB-1 to AB-6) ranging from 12 to 18 residues covering the most hydrophilic regions of the protein, and two more peptides (AB-7 and AB-8) representing its repetitive sequences. We found that all eight constructs bound an appreciable amount of antibody in sera from a large proportion of P. falciparum malaria patients; two of these peptides (AB-1 and AB-3) also elicited a strong proliferation response in peripheral blood mononuclear cells from all 11 human subjects recovering from malaria. When used as carrier-free immunogens, six peptides induced a strong, boostable, immunoglobulin G-type antibody response in rabbits, indicating the presence of both B-cell determinants and T-helper-cell epitopes in these six constructs. These antibodies specifically cross-reacted with the parasite protein(s) in an immunoblot and in an immunofluorescence assay. In another immunoblot, rabbit antipeptide sera also recognized recombinant fragments of ABRA expressed in bacteria. More significantly, rabbit antibodies against two constructs (AB-1 and AB-5) inhibited the merozoite reinvasion of human erythrocytes in vitro up to approximately 90%. These results favor further studies so as to determine possible inclusion of these two constructs in a multicomponent subunit vaccine against asexual blood stages of P. falciparum.
Figures



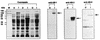

References
-
- Alonso P L, Smith T, Armstrong Schellenberg J R M, Masanja H, Mwankusye S, Urassa H, Bastos de Azevedo I, Chongela J, Kobero S, Menendez C, Hurt N, Thomas M C, Lyimo E, Weiss N A, Hayes R, Kitua A Y, Lopez M C, Kilama W L, Teuscher T, Tanner M. Randomised trial of efficacy of SPf66 vaccine against Plasmodium falciparum malaria in children in southern Tanzania. Lancet. 1994;344:1175–1181. - PubMed
-
- Anders R F. Multiple cross-reactivities amongst antigens of Plasmodium falciparum impair the development of protective immunity against malaria. Parasite Immunol. 1986;8:529–539. - PubMed
-
- Anders R F, Smythe J A. Polymorphic antigens in Plasmodium falciparum. Blood. 1989;74:1865–1875. - PubMed
-
- Banyal H S, Misra G C, Gupta C M, Dutta G P. Involvement of malaria proteases in the interaction between the parasite and the host erythrocyte in Plasmodium knowlesi infections. J Parasitol. 1980;67:623–626. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

