In vivo binding of immunoglobulin M to the surfaces of Babesia bigemina-infected erythrocytes
- PMID: 9596768
- PMCID: PMC108290
- DOI: 10.1128/IAI.66.6.2922-2927.1998
In vivo binding of immunoglobulin M to the surfaces of Babesia bigemina-infected erythrocytes
Abstract
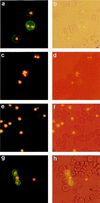
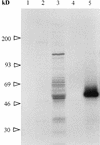
Babesia bigemina infection of mature bovine erythrocytes results in new proteins specifically exposed on the parasitized cell surface. Monoclonal antibody (MAb) 64/32 binds a protein, designated p94, on B. bigemina-infected erythrocytes but not on either uninfected or B. bovis-parasitized erythrocytes. However, p94 was not encoded by B. bigemina and was not a parasite-modified erythrocyte membrane protein. In contrast, we showed that p94 could be eluted from the infected erythrocyte surface and was identified as specifically bound immunoglobulin M (IgM) heavy chain for the following reasons: (i) MAb 64/32 bound a reduced molecule of 94 kDa in both infected erythrocyte lysates and normal bovine serum; (ii) MAb 64/32 bound a 94-kDa molecule in reduced preparations of purified IgM; (iii) an anti-bovine mu heavy-chain MAb, BIg73, reacted specifically with the surface of infected erythrocytes and bound the 94-kDa molecule in lysates of infected erythrocytes, normal bovine serum, and purified IgM; and (iv) immunoprecipitation of infected erythrocyte lysates with MAb 64/32 depleted the 94-kDa antigen bound by anti-mu MAb BIg73 and vice versa. Binding of IgM to the infected erythrocyte surface was detected in vivo early in acute parasitemia and occurred during both the trophozoite and merozoite stages of intraerythrocytic parasitism. The common feature of IgM binding to the parasitized erythrocyte surface among otherwise genetically and antigenically distinct B. bigemina strains is suggestive of an advantageous role in parasite survival in vivo.
Figures


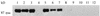
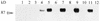
Similar articles
-
Babesia bigemina: identification of B cell epitopes associated with parasitized erythrocytes.Exp Parasitol. 1995 Dec;81(4):491-500. doi: 10.1006/expr.1995.1142. Exp Parasitol. 1995. PMID: 8542990
-
Monoclonal antibody to a conserved epitope on proteins encoded by Babesia bigemina and present on the surface of intact infected erythrocytes.Infect Immun. 1995 Sep;63(9):3507-13. doi: 10.1128/iai.63.9.3507-3513.1995. Infect Immun. 1995. PMID: 7543884 Free PMC article.
-
Identification of Babesia bigemina infected erythrocyte surface antigens containing epitopes conserved among strains.Parasite Immunol. 1994 Mar;16(3):119-27. doi: 10.1111/j.1365-3024.1994.tb00331.x. Parasite Immunol. 1994. PMID: 8208585
-
Tick-borne diseases in ruminants of Central and Southern Italy: epidemiology and case reports.Parassitologia. 1999 Sep;41 Suppl 1:95-100. Parassitologia. 1999. PMID: 11071553 Review.
-
Delivery of drugs bound to erythrocytes: new avenues for an old intravascular carrier.Ther Deliv. 2015 Jul;6(7):795-826. doi: 10.4155/tde.15.34. Ther Deliv. 2015. PMID: 26228773 Free PMC article. Review.
Cited by
-
Unravelling the cellular and molecular pathogenesis of bovine babesiosis: is the sky the limit?Int J Parasitol. 2019 Feb;49(2):183-197. doi: 10.1016/j.ijpara.2018.11.002. Epub 2019 Jan 26. Int J Parasitol. 2019. PMID: 30690089 Free PMC article. Review.
-
Nonspecific immunoglobulin M binding and chondroitin sulfate A binding are linked phenotypes of Plasmodium falciparum isolates implicated in malaria during pregnancy.Infect Immun. 2003 Aug;71(8):4767-71. doi: 10.1128/IAI.71.8.4767-4771.2003. Infect Immun. 2003. PMID: 12874359 Free PMC article.
-
Investigating the function of Fc-specific binding of IgM to Plasmodium falciparum erythrocyte membrane protein 1 mediating erythrocyte rosetting.Cell Microbiol. 2015 Jun;17(6):819-31. doi: 10.1111/cmi.12403. Epub 2015 Jan 28. Cell Microbiol. 2015. PMID: 25482886 Free PMC article.
-
Assessment of Babesia bovis 6cys A and 6cys B as components of transmission blocking vaccines for babesiosis.Parasit Vectors. 2021 Apr 20;14(1):210. doi: 10.1186/s13071-021-04712-7. Parasit Vectors. 2021. PMID: 33879245 Free PMC article.
-
Babesiosis.Clin Microbiol Rev. 2000 Jul;13(3):451-69. doi: 10.1128/CMR.13.3.451. Clin Microbiol Rev. 2000. PMID: 10885987 Free PMC article. Review.
References
-
- Adams J H, Hudson D E, Torii M, Ward G E, Wellems T E, Aikawa M, Miller L H. The Duffy receptor family of Plasmodium knowlesi is located within the micronemes of invasive malaria merozoites. Cell. 1990;63:141–153. - PubMed
-
- Aikawa M, Rabbege J, Uni S, Ristic M, Miller L H. Structural alteration of the membrane of the erythrocytes infected with Babesia bovis. Am J Trop Med Hyg. 1985;34:45–49. - PubMed
-
- Baruch D, Pasloske B L, Singh H B, Bi X, Ma X C, Feldman M, Taraschi T F, Howard R J. Cloning the P. falciparum gene encoding PfEMP1, a malarial variant antigen and adherence receptor on the surface of parasitized human erythrocytes. Cell. 1995;82:77–87. - PubMed
-
- Berendt A R, Ferguson D J P, Gardner J, Turner G, Rowe A, McCormick C, Roberts D, Craig A, Pinches R, Elford B C, Newbold C I. Molecular mechanisms of sequestration in malaria. Parasitol. 1990;108:S19–S28. - PubMed
-
- Crandall I, Guthrie N, Sherman I W. Plasmodium falciparum: sera of individuals living in a malaria-endemic region recognize peptide motifs of the human erythrocyte anion transport protein. Am J Trop Med Hyg. 1995;52:450–452. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

