Potentiation of GABAergic synaptic transmission by AMPA receptors in mouse cerebellar stellate cells: changes during development
- PMID: 9596802
- PMCID: PMC2230994
- DOI: 10.1111/j.1469-7793.1998.817bm.x
Potentiation of GABAergic synaptic transmission by AMPA receptors in mouse cerebellar stellate cells: changes during development
Abstract
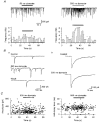
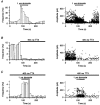
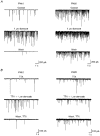
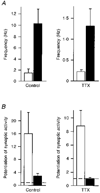
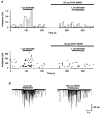
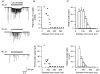
1. The effects of low concentrations of domoate, an agonist at both alpha-amino-3-hydroxy-5-methylisoxazole-4-propionate and kainate receptors (AMPARs and KARs, respectively), were investigated in stellate cells in slices of mouse cerebellum at two developmental stages (postnatal day (PN) 11-13 and PN21-25). 2. Low concentrations of domoate enhanced the frequency of miniature IPSCs (mIPSCs) recorded in the presence of tetrodotoxin (TTX) at PN11-13 but not at PN21-25. 3. The effects of low concentrations of domoate on synaptic activity were probably mediated by the activation of AMPARs and not KARs, since they were blocked by GYKI 53655 (LY300168), a selective AMPAR antagonist. 4. Domoate increased mIPSC frequency in part by activation of presynaptic voltage-dependent Ca2+ channels since potentiation was reduced by 60 % in the presence of Cd2+. AMPARs in stellate cells were found to be permeable to Ca2+. The residual potentiation in the presence of Cd2+ could thus be due to a direct entry of Ca2+ through AMPAR channels. 5. In the presence of TTX, potentiation of synaptic activity by focal application of domoate was not restricted to the region of the cell body, but was observed within distances of 120 micro(m). These experiments also revealed a strong spatial correlation between the location of the presynaptic effects of domoate and the activation of postsynaptic AMPARs. 6. Our data show a developmentally regulated presynaptic potentiation of synaptic transmission between cerebellar interneurones mediated by AMPARs. We discuss the possibility that the developmental switch could be due to a shift in the localization of AMPARs from the axonal to the somato-dendritic compartment.
Figures
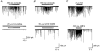


References
-
- Altman J, Bayer SA. Development of the Cerebellar System in Relation to its Evolution, Structure and Functions. Boca Raton, FL, USA: CRC Press; 1997.
-
- Ankri N, Legendre P, Faber DS, Korn H. Automatic detection of spontaneous synaptic responses in central neurones. Journal of Neuroscience Methods. 1994;52:87–100. 10.1016/0165-0270(94)90060-4. - DOI - PubMed
-
- Bettler B, Egebjerg J, Sharma G, Pecht G, Hermans-Borgmeyer I, Moll C, Stevens CF, Heinemann S. Cloning of a putative glutamate receptor: a low affinity kainate receptor subunit. Neuron. 1992;8:257–265. - PubMed
-
- Bettler B, Mulle C. AMPA and kainate receptors. Neuropharmacology. 1995;34:123–139. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

