Kainate receptor-mediated inhibition of presynaptic Ca2+ influx and EPSP in area CA1 of the rat hippocampus
- PMID: 9596803
- PMCID: PMC2230988
- DOI: 10.1111/j.1469-7793.1998.833bm.x
Kainate receptor-mediated inhibition of presynaptic Ca2+ influx and EPSP in area CA1 of the rat hippocampus
Abstract
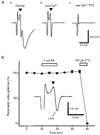
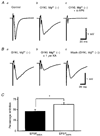
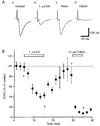
1. The effect of a low concentration (1 microM) of kainate (kainic acid; KA) on presynaptic calcium (Ca2+) influx at the Schaffer collateral-commissural (SCC) synapse was examined in rat hippocampal slices. 2. Following selective loading of the presynaptic terminals with the fluorescent Ca2+ indicator rhod-2 AM, transient increases in the presynaptic Ca2+ concentration (pre[Ca2+]t) and field excitatory postsynaptic potentials (EPSPs) evoked by electrical stimulation of the SCC pathway were recorded simultaneously. 3. Bath application of 1 microM KA reversibly suppressed field EPSPs and pre[Ca2+]t to 37.7 +/- 4.0 % and 72.9 +/- 2.4 % of control, respectively. Excitatory postsynaptic currents (EPSCs) recorded with the use of the whole-cell patch-clamp technique were also suppressed by 1 microM KA to 42.6 +/- 6.3 % of control. A quantitative analysis of the decreases in pre[Ca2+]t and the amplitude of field EPSP during KA application suggests that KA inhibits transmission primarily by reducing the pre[Ca2+]t. 4. Consistent with a presynaptic site for these effects, paired-pulse facilitation (PPF) was enhanced by 1 microM KA. 5. A substantial KA-induced suppression of NMDA receptor-mediated EPSPs was detected when AMPA receptors were blocked by the AMPA receptor-selective antagonist GYKI 52466 (100 microM). 6. The suppressive effect of KA on field EPSPs and pre[Ca2+]t was antagonized by the KA antagonist NS-102 (10 microM). 7. These results suggest that the presynaptic inhibitory action of KA at the hippocampal CA1 synapse is primarily due to the inhibition of Ca2+ influx into the presynaptic terminals.
Figures




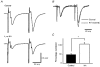
References
-
- Bettler B, Mulle C. Neurotransmitter receptors II. AMPA and kainate receptors. Neuropharmacology. 1995;34:123–139. - PubMed
-
- Castillo P E, Malenka R C, Nicoll R A. Kainate receptors mediate a slow postsynaptic current in hippocampal CA3 neurons. Nature. 1997;388:182–186. 10.1038/40645. - DOI - PubMed
-
- Chittajallu R, Vignes M, Dev K K, Barnes J M, Collingridge G L, Henley J M. Regulation of glutamate release by presynaptic kainate receptors in the hippocampus. Nature. 1996;379:78–81. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

