Architecture and mechanism of the light-harvesting apparatus of purple bacteria
- PMID: 9600895
- PMCID: PMC34498
- DOI: 10.1073/pnas.95.11.5935
Architecture and mechanism of the light-harvesting apparatus of purple bacteria
Abstract
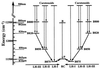
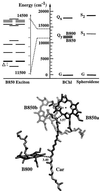
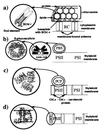
Photosynthetic organisms fuel their metabolism with light energy and have developed for this purpose an efficient apparatus for harvesting sunlight. The atomic structure of the apparatus, as it evolved in purple bacteria, has been constructed through a combination of x-ray crystallography, electron microscopy, and modeling. The detailed structure and overall architecture reveals a hierarchical aggregate of pigments that utilizes, as shown through femtosecond spectroscopy and quantum physics, elegant and efficient mechanisms for primary light absorption and transfer of electronic excitation toward the photosynthetic reaction center.
Figures





References
-
- Hu X, Schulten K. Physics Today. 1997;50:28–34.
-
- Duysens L N M. Ph.D. thesis. The Netherlands: Utrecht; 1952.
-
- Cogdell R, Fyfe P, Barrett S, Prince S, Freer A, Isaacs N, McGlynn P, Hunter C. Photosynth Res. 1996;48:55–63. - PubMed
-
- Krauss N, Schubert W-D, Klukas O, Fromme P, Witt H T, Saenger W. Nat Struct Biol. 1996;3:965–973. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

