Nonneuronal isoforms of STOP protein are responsible for microtubule cold stability in mammalian fibroblasts
- PMID: 9600916
- PMCID: PMC27584
- DOI: 10.1073/pnas.95.11.6055
Nonneuronal isoforms of STOP protein are responsible for microtubule cold stability in mammalian fibroblasts
Abstract
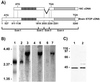
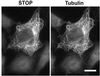
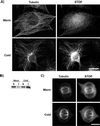
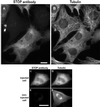
A number of cycling mammalian cells, such as NIH 3T3, contain abundant subsets of cold-stable microtubules. The origin of such microtubule stabilization in nonneuronal cells is unknown. We have previously described a neuronal protein, stable tubule-only polypeptide (STOP), that binds to microtubules and induces cold stability. We find that NIH 3T3 fibroblasts contain a major 42-kDa isoform of STOP (fibroblastic STOP, F-STOP). F-STOP contains the central repeats characteristic of brain STOP but shows extensive deletions of N- and C-terminal protein domains that are present in brain STOP. These deletions arise from differences in STOP RNA splicing. Despite such deletions, F-STOP has full microtubule stabilizing activity. F-STOP accumulates on cold-stable microtubules of interphase arrays and is present on stable microtubules within the mitotic spindle of NIH 3T3 cells. STOP inhibition by microinjection of affinity-purified STOP central repeat antibodies into NIH 3T3 cells abolishes both interphase and spindle microtubule cold stability. Similar results were obtained with Rat2 cells. These results show that STOP proteins have nonneuronal isoforms that are responsible for the microtubule cold stability observed in mammalian fibroblasts.
Figures

References
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

