Differential expression of dystrophin isoforms and utrophin during dibutyryl-cAMP-induced morphological differentiation of rat brain astrocytes
- PMID: 9600931
- PMCID: PMC27600
- DOI: 10.1073/pnas.95.11.6139
Differential expression of dystrophin isoforms and utrophin during dibutyryl-cAMP-induced morphological differentiation of rat brain astrocytes
Abstract
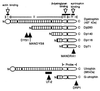
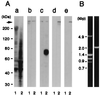
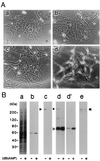
We have identified isoforms of dystrophin and utrophin, a dystrophin homologue, expressed in astrocytes and examined their expression patterns during dibutyryl-cAMP (dBcAMP)-induced morphological differentiation of astrocytes. Immunoblot and immunocytochemical analyses showed that full-length-type dystrophin (427 kDa), utrophin (395 kDa), and Dp71 (75 kDa), a small-type dystrophin isoform, were coexpressed in cultured nondifferentiated rat brain astrocytes and were found to be located in the cell membrane. During morphological differentiation of the astrocytes induced by 1 mM dBcAMP, the amount of Dp71 markedly increased, whereas that of dystrophin and utrophin decreased. Northern blot analyses revealed that dBcAMP regulates the mRNA levels of Dp71 and dystrophin but not that of utrophin. dBcAMP slightly increased the amount of the beta-dystroglycan responsible for anchoring dystrophin isoforms and utrophin to the cell membrane. Immunocytochemical analyses showed that most utrophin was observed in the cytoplasmic area during astrocyte differentiation, whereas Dp71 was found along the cell membrane of the differentiated astrocytes. These findings suggest that most of the dystrophin/utrophin-dystroglycan complex on cell membrane in cultured astrocytes was replaced by the Dp71-dystroglycan complex during morphological differentiation. The cell biological roles of Dp71 are discussed.
Figures

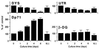
References
-
- Ozawa E, Yoshida M, Suzuki A, Mizuno Y, Hagiwara Y, Noguchi S. Hum Mol Genet. 1995;4:1711–1716. - PubMed
-
- Sadoulet-Puccio H M, Kunkel L M. Brain Pathol. 1996;6:25–35. - PubMed
-
- Love D R, Hill D F, Dickson G, Spurr N K, Byth B C, Marsden R F, Walsh F S, Edwards Y H, Davies K E. Nature (London) 1989;339:55–58. - PubMed
-
- Blake D J, Love D R, Tinsley J, Morris G E, Turley H, Gatter K, Dickson G, Edwards Y H, Davies K E. Hum Mol Genet. 1992;1:103–109. - PubMed
-
- Tinsley J M, Blake D J, Roche A, Fairbrother U, Riss J, Byth B C, Knight A E, Kendrick-Jones J, Suthers G K, Love D R, Edwards Y H, Davies K E. Nature (London) 1992;360:591–593. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources

