Tyrosine kinase-dependent activation of a chloride channel in CD95-induced apoptosis in T lymphocytes
- PMID: 9600936
- PMCID: PMC27614
- DOI: 10.1073/pnas.95.11.6169
Tyrosine kinase-dependent activation of a chloride channel in CD95-induced apoptosis in T lymphocytes
Abstract
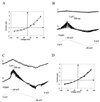
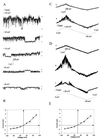
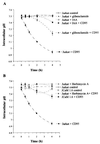
CD95/Fas/APO-1 mediated apoptosis is an important mechanism in the regulation of the immune response. Here, we show that CD95 receptor triggering activates an outwardly rectifying chloride channel (ORCC) in Jurkat T lymphocytes. Ceramide, a lipid metabolite synthesized upon CD95 receptor triggering, also induces activation of ORCC in cell-attached patch clamp experiments. Activation is mediated by Src-like tyrosine kinases, because it is abolished by the tyrosine kinase inhibitor herbimycin A or by genetic deficiency of p56lck. In vitro incubation of excised patches with purified p56lck results in activation of ORCC, which is partially reversed upon addition of anti-phosphotyrosine antibody. Inhibition of ORCC by four different drugs correlates with a 30-65% inhibition of apoptosis. Intracellular acidification observed upon CD95 triggering is abolished by inhibition of either ORCC or p56lck. The results suggest that tyrosine kinase-mediated activation of ORCC may play a role in CD95-induced cell death in T lymphocytes.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

