Hydrolysis of GTP on rab11 is required for the direct delivery of transferrin from the pericentriolar recycling compartment to the cell surface but not from sorting endosomes
- PMID: 9600939
- PMCID: PMC27621
- DOI: 10.1073/pnas.95.11.6187
Hydrolysis of GTP on rab11 is required for the direct delivery of transferrin from the pericentriolar recycling compartment to the cell surface but not from sorting endosomes
Abstract
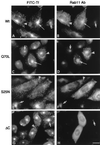
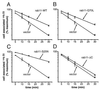
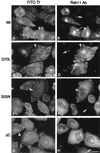
Rab11 is a small GTP-binding protein that in cultured mammalian cells has been shown to be concentrated in the pericentriolar endosomal recycling compartment and to play a key role in passage of the recycling transferrin receptor through that compartment [Ullrich, O., Reinsch, S., Urbé, S., Zerial, M. & Parton, R. G. (1996) J. Cell Biol. 135, 913-924]. To obtain insights into the site(s) of action of rab11 within the recycling pathway, we have now compared the effects on recycling at 37 degreesC of overexpression of wild-type rab11 and various mutant forms of this protein in cells that had been loaded with transferrin at either 37 degreesC or 16 degreesC. We show that incubation at 16 degreesC blocks passage of endocytosed transferrin into the recycling compartment and that, whereas the rab11 dominant negative mutant form (S25N) inhibits transferrin recycling after interiorization at either temperature, the wild-type rab11 and constitutively active mutant (Q70L) have no inhibitory effect on the recycling of molecules that were interiorized at 16 degreesC. This differential inhibitory effect shows that two distinct pathways for recycling are followed by the bulk of the transferrin molecules interiorized at the two different temperatures. The incapacity of the constitutively active form of rab11 (Q70L) to inhibit recycling of molecules interiorized at 16 degreesC is consistent with their recycling taking place directly from sorting endosomes, in a process that does not require hydrolysis of GTP on rab11. The fact that the dominant negative (S25N) form of rab11 inhibits recycling of molecules interiorized at both temperatures indicates that activation of rab11 by GTP is required for exit of transferrin from sorting endosomes, regardless of whether this exit is toward the recycling compartment or directly to the plasma membrane.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

