Male fitness increases when females are eliminated from gene pool: implications for the Y chromosome
- PMID: 9600945
- PMCID: PMC27633
- DOI: 10.1073/pnas.95.11.6217
Male fitness increases when females are eliminated from gene pool: implications for the Y chromosome
Abstract
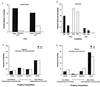
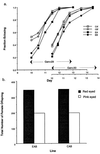
Because the two sexes share a common gene pool while performing many different biological functions, mutations benefiting one sex may not accumulate due to counter selection in the other sex. In these experiments 99% of a haploid genome of Drosophila melanogaster was constrained to segregate like a male-limited Y chromosome for 41 generations, thereby eliminating potential counter selection in females. The synthetic Y chromosomes rapidly accumulated genetic variation that increased male fitness and decreased female fitness. The survival and fertility of females declined when they were mated to males expressing the synthetic Y chromosomes. These results suggests that opposing selection between the sexes may substantially interfere with sex-specific adaptation. They also demonstrate how intersexual evolutionary conflict can lead to perpetual degeneration of the Y via genetic hitchhiking of deleterious mutations.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

