Wheat grain hardness results from highly conserved mutations in the friabilin components puroindoline a and b
- PMID: 9600953
- PMCID: PMC27651
- DOI: 10.1073/pnas.95.11.6262
Wheat grain hardness results from highly conserved mutations in the friabilin components puroindoline a and b
Abstract
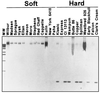
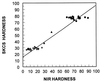
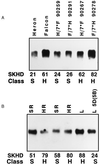
"Soft" and "hard" are the two main market classes of wheat (Triticum aestivum L.) and are distinguished by expression of the Hardness gene. Friabilin, a marker protein for grain softness (Ha), consists of two proteins, puroindoline a and b (pinA and pinB, respectively). We previously demonstrated that a glycine to serine mutation in pinB is linked inseparably to grain hardness. Here, we report that the pinB serine mutation is present in 9 of 13 additional randomly selected hard wheats and in none of 10 soft wheats. The four exceptional hard wheats not containing the serine mutation in pinB express no pinA, the remaining component of the marker protein friabilin. The absence of pinA protein was linked inseparably to grain hardness among 44 near-isogenic lines created between the soft variety Heron and the hard variety Falcon. Both pinA and pinB apparently are required for the expression of grain softness. The absence of pinA protein and transcript and a glycine-to-serine mutation in pinB are two highly conserved mutations associated with grain hardness, and these friabilin genes are the suggested tightly linked components of the Hardness gene. A previously described grain hardness related gene termed "GSP-1" (grain softness protein) is not controlled by chromosome 5D and is apparently not involved in grain hardness. The association of grain hardness with mutations in both pinA or pinB indicates that these two proteins alone may function together to effect grain softness. Elucidation of the molecular basis for grain hardness opens the way to understanding and eventually manipulating this wheat endosperm property.
Figures


References
-
- Morris C F, Rose S P. Cereal Grain Quality. New York: Chapman and Hall; 1996. pp. 3–54.
-
- Symes K J. Aust J Agric Res. 1965;16:113–123.
-
- Baker R J. Crop Sci. 1977;17:960–962.
-
- Mattern P J, Morris R, Schmidt J W, Johnson V A. Proceedings of the 4th International Wheat Genetics Symposium. Columbia, MO: Univ. Missouri; 1973. pp. 703–707.
-
- Law C N, Young C F, Brown J W S, Snape J W, Worland J W. Seed Protein Improvement by Nuclear Techniques. Vienna, Austria: International Atomic Energy Agency; 1978. pp. 483–502.
LinkOut - more resources
Full Text Sources
Other Literature Sources

