Posttranscriptional regulation of urokinase plasminogen activator receptor messenger RNA levels by leukocyte integrin engagement
- PMID: 9600959
- PMCID: PMC27663
- DOI: 10.1073/pnas.95.11.6296
Posttranscriptional regulation of urokinase plasminogen activator receptor messenger RNA levels by leukocyte integrin engagement
Abstract
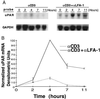
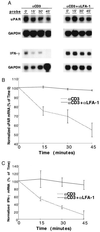
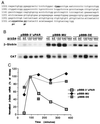
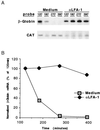
As an adhesion receptor, the beta2 integrin lymphocyte function-associated antigen-1 (LFA-1) contributes a strong adhesive force to promote T lymphocyte recirculation and interaction with antigen-presenting cells. As a signaling molecule, LFA-1-mediates transmembrane signaling, which leads to the generation of second messengers and costimulation resulting in T cell activation. We recently have demonstrated that, in costimulatory fashion, LFA-1 activation promotes the induction of T cell membrane urokinase plasminogen activator receptor (uPAR) and that this induced uPAR is functional. To investigate the mechanism(s) of this induction, we used the RNA polymerase II inhibitor 5, 6-dichloro-1-beta-D-ribobenzimidazole and determined that uPAR mRNA degradation is delayed by LFA-1 activation. Cloning of the wild-type, deleted and mutated 3'-untranslated region of the uPAR cDNA into a serum-inducible rabbit beta-globin cDNA reporter construct revealed that the AU-rich elements and, in particular the nonameric UUAUUUAUU sequence, are crucial cis-acting elements in uPAR mRNA degradation. Experiments in which Jurkat T cells were transfected with reporter constructs demonstrated that LFA-1 engagement was able to stabilize the unstable reporter mRNA containing the uPAR 3'-untranslated region. Our study reveals a consequence of adhesion receptor-mediated signaling in T cells, which is potentially important in the regulation of T cell activation, including production of cytokines and expression of proto-oncogenes, many of which are controlled through 3' AU-rich elements.
Figures

References
-
- Hynes R O. Cell. 1992;69:11–25. - PubMed
-
- Schwartz R H. Cell. 1992;71:1065–1068. - PubMed
-
- Fan S-T, Brian A A, Lollo B A, Mackman N, Shen N L, Edington T S. Cell Immunol. 1993;148:48–59. - PubMed
-
- Damle N K, Klussman K, Leytze G, Myrdal S, Aruffo A, Ledbetter J A, Linsley P S. J Immunol. 1994;152:2686– 2697. - PubMed
-
- Van Seventer G A, Shimizu Y, Horgan K J, Shaw S. J Immunol. 1990;144:4579–4586. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

