In vivo detection and imaging of phosphatidylserine expression during programmed cell death
- PMID: 9600968
- PMCID: PMC27696
- DOI: 10.1073/pnas.95.11.6349
In vivo detection and imaging of phosphatidylserine expression during programmed cell death
Abstract
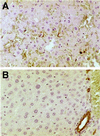
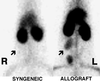
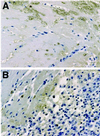
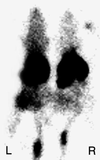
One of the earliest events in programmed cell death is the externalization of phosphatidylserine, a membrane phospholipid normally restricted to the inner leaflet of the lipid bilayer. Annexin V, an endogenous human protein with a high affinity for membrane bound phosphatidylserine, can be used in vitro to detect apoptosis before other well described morphologic or nuclear changes associated with programmed cell death. We tested the ability of exogenously administered radiolabeled annexin V to concentrate at sites of apoptotic cell death in vivo. After derivatization with hydrazinonicotinamide, annexin V was radiolabeled with technetium 99m. In vivo localization of technetium 99m hydrazinonicotinamide-annexin V was tested in three models: fuminant hepatic apoptosis induced by anti-Fas antibody injection in BALB/c mice; acute rejection in ACI rats with transplanted heterotopic PVG cardiac allografts; and cyclophosphamide treatment of transplanted 38C13 murine B cell lymphomas. External radionuclide imaging showed a two- to sixfold increase in the uptake of radiolabeled annexin V at sites of apoptosis in all three models. Immunohistochemical staining of cardiac allografts for exogenously administered annexin V revealed intense staining of numerous myocytes at the periphery of mononuclear infiltrates of which only a few demonstrated positive apoptotic nuclei by the terminal deoxynucleotidyltransferase-mediated UTP end labeling method. These results suggest that radiolabeled annexin V can be used in vivo as a noninvasive means to detect and serially image tissues and organs undergoing programmed cell death.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

