Abnormal transport along the lysosomal pathway in mucolipidosis, type IV disease
- PMID: 9600972
- PMCID: PMC27719
- DOI: 10.1073/pnas.95.11.6373
Abnormal transport along the lysosomal pathway in mucolipidosis, type IV disease
Abstract
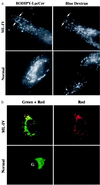
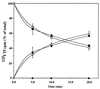
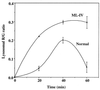
Mucolipidosis, type IV (ML-IV) is an autosomal recessive storage disease that is characterized by lysosomal accumulation of sphingolipids, phospholipids, and acid mucopolysaccharides. Unlike most other storage diseases, the lysosomal hydrolases participating in the catabolism of the stored molecules appear to be normal. In the present study, we examined the hypothesis that the ML-IV phenotype might arise from abnormal transport along the lysosomal pathway. By using various markers for endocytosis, we found that plasma membrane internalization and recycling were nearly identical in ML-IV and normal fibroblasts. A fluorescent analog of lactosylceramide (LacCer) was used to study plasma membrane lipid internalization and subsequent transport. Lipid internalization at 19 degreesC was similar in both cell types; however, 40-60 min after raising the temperature to 37 degreesC, the fluorescent lipid accumulated in the lysosomes of ML-IV cells but was mainly concentrated at the Golgi complex of normal fibroblasts. Biochemical studies demonstrated that at these time points, hydrolysis of the lipid analog was minimal ( approximately 7%) in both cell types. A fluorescence ratio imaging assay was developed to monitor accumulation of fluorescent LacCer in the lysosomes and showed that the apparent concentration of the lipid increased more rapidly and to a greater extent in ML-IV cells than in normal fibroblasts. By 60 min, LacCer apparently decreased in the lysosomes of normal fibroblasts but not in ML-IV cells, suggesting that lipid efflux from the lysosomes was also impaired. These results demonstrate that there is a defect in ML-IV fibroblasts that affects membrane sorting and/or late steps of endocytosis.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

