Diffusible, nonfibrillar ligands derived from Abeta1-42 are potent central nervous system neurotoxins
- PMID: 9600986
- PMCID: PMC27787
- DOI: 10.1073/pnas.95.11.6448
Diffusible, nonfibrillar ligands derived from Abeta1-42 are potent central nervous system neurotoxins
Abstract
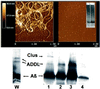
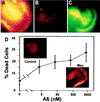
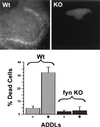
Abeta1-42 is a self-associating peptide whose neurotoxic derivatives are thought to play a role in Alzheimer's pathogenesis. Neurotoxicity of amyloid beta protein (Abeta) has been attributed to its fibrillar forms, but experiments presented here characterize neurotoxins that assemble when fibril formation is inhibited. These neurotoxins comprise small diffusible Abeta oligomers (referred to as ADDLs, for Abeta-derived diffusible ligands), which were found to kill mature neurons in organotypic central nervous system cultures at nanomolar concentrations. At cell surfaces, ADDLs bound to trypsin-sensitive sites and surface-derived tryptic peptides blocked binding and afforded neuroprotection. Germ-line knockout of Fyn, a protein tyrosine kinase linked to apoptosis and elevated in Alzheimer's disease, also was neuroprotective. Remarkably, neurological dysfunction evoked by ADDLs occurred well in advance of cellular degeneration. Without lag, and despite retention of evoked action potentials, ADDLs inhibited hippocampal long-term potentiation, indicating an immediate impact on signal transduction. We hypothesize that impaired synaptic plasticity and associated memory dysfunction during early stage Alzheimer's disease and severe cellular degeneration and dementia during end stage could be caused by the biphasic impact of Abeta-derived diffusible ligands acting upon particular neural signal transduction pathways.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

