The 3' untranslated region of a rice alpha-amylase gene functions as a sugar-dependent mRNA stability determinant
- PMID: 9601003
- PMCID: PMC27866
- DOI: 10.1073/pnas.95.11.6543
The 3' untranslated region of a rice alpha-amylase gene functions as a sugar-dependent mRNA stability determinant
Abstract
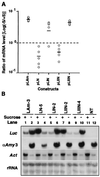
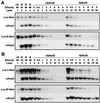
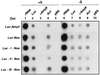
In plants, sugar feedback regulation provides a mechanism for control of carbohydrate allocation and utilization among tissues and organs. The sugar repression of alpha-amylase gene expression in rice provides an ideal model for studying the mechanism of sugar feedback regulation. We have shown previously that sugar repression of alpha-amylase gene expression in rice suspension cells involves control of both transcription rate and mRNA stability. The alpha-amylase mRNA is significantly more stable in sucrose-starved cells than in sucrose-provided cells. To elucidate the mechanism of sugar-dependent mRNA turnover, we have examined the effect of alphaAmy3 3' untranslated region (UTR) on mRNA stability by functional analyses in transformed rice suspension cells. We found that the entire alphaAmy3 3' UTR and two of its subdomains can independently mediate sugar-dependent repression of reporter mRNA accumulation. Analysis of reporter mRNA half-lives demonstrated that the entire alphaAmy3 3' UTR and the two subdomains each functioned as a sugar-dependent destabilizing determinant in the turnover of mRNA. Nuclear run-on transcription analysis further confirmed that the alphaAmy3 3' UTR and the two subdomains did not affect the transcription rate of promoter. The identification of sequence elements in the alpha-amylase mRNA that dictate the differential stability has very important implications for the study of sugar-dependent mRNA decay mechanisms.
Figures


References
-
- Sheen J. Photosynth Res. 1994;39:427–438. - PubMed
-
- Yu S-M, Lee Y-C, Fang S-C, Chan M-T, Hwa S-F, Liu L-F. Plant Mol Biol. 1996;30:1277–1289. - PubMed
-
- Koch K E. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:509–540. - PubMed
-
- Yu S-M, Kuo Y-H, Sheu G, Sheu Y-J, Liu L-F. J Biol Chem. 1991;266:21131–21137. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

