Reduction of soluble iron and reductive dissolution of ferric iron-containing minerals by moderately thermophilic iron-oxidizing bacteria
- PMID: 9603832
- PMCID: PMC106296
- DOI: 10.1128/AEM.64.6.2181-2186.1998
Reduction of soluble iron and reductive dissolution of ferric iron-containing minerals by moderately thermophilic iron-oxidizing bacteria
Abstract
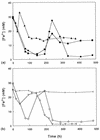
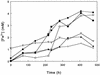
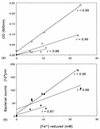
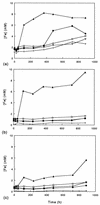
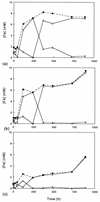
Five moderately thermophilic iron-oxidizing bacteria, including representative strains of the three classified species (Sulfobacillus thermosulfidooxidans, Sulfobacillus acidophilus, and Acidimicrobium ferrooxidans), were shown to be capable of reducing ferric iron to ferrous iron when they were grown under oxygen limitation conditions. Iron reduction was most readily observed when the isolates were grown as mixotrophs or heterotrophs with glycerol as an electron donor; in addition, some strains were able to couple the oxidation of tetrathionate to the reduction of ferric iron. Cycling of iron between the ferrous and ferric states was observed during batch culture growth in unshaken flasks incubated under aerobic conditions, although the patterns of oxidoreduction of iron varied in different species of iron-oxidizing moderate thermophiles and in strains of a single species (S. acidophilus). All three bacterial species were able to grow anaerobically with ferric iron as a sole electron acceptor; the growth yields correlated with the amount of ferric iron reduced when the isolates were grown in the absence of oxygen. One of the moderate thermophiles (identified as a strain of S. acidophilus) was able to bring about the reductive dissolution of three ferric iron-containing minerals (ferric hydroxide, jarosite, and goethite) when it was grown under restricted aeration conditions with glycerol as a carbon and energy source. The significance of iron reduction by moderately thermophilic iron oxidizers in both environmental and applied contexts is discussed.
Figures

References
-
- Atkinson R J, Posner A M, Quirk J P. Crystal nucleation and growth in hydrolysing iron(III) chloride solutions. Clays Clay Miner. 1977;25:9–56.
-
- Bridge, T. A. M., and D. B. Johnson. Unpublished data.
-
- Brierley J A, Norris P R, Kelly D P, Le Roux N W. Characteristics of a moderately thermophilic and acidophilic iron-oxidizing Thiobacillus. Eur J Appl Microbiol. 1978;5:291–299.
-
- Clark D A, Norris P R. Acidimicrobium ferrooxidans gen. nov., sp. nov.: mixed-culture ferrous iron oxidation with Sulfobacillus species. Microbiology. 1996;141:785–790. - PubMed
LinkOut - more resources
Full Text Sources

