Isolation of a bacterial strain with the ability to utilize the sulfonated azo compound 4-carboxy-4'-sulfoazobenzene as the sole source of carbon and energy
- PMID: 9603860
- PMCID: PMC106324
- DOI: 10.1128/AEM.64.6.2315-2317.1998
Isolation of a bacterial strain with the ability to utilize the sulfonated azo compound 4-carboxy-4'-sulfoazobenzene as the sole source of carbon and energy
Abstract
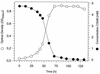
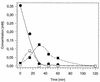
A bacterial strain (strain S5) which grows aerobically with the sulfonated azo compound 4-carboxy-4'-sulfoazobenzene as the sole source of carbon and energy was isolated. This strain was obtained by continuous adaptation of "Hydrogenophaga palleronii" S1, which has the ability to grow aerobically with 4-aminobenzenesulfonate. Strain S5 probably cleaves 4-carboxy-4'-sulfoazobenzene reductively under aerobic conditions to 4-aminobenzoate and 4-aminobenzene-sulfonate, which are mineralized by previously established degradation pathways.
Figures

References
-
- Bayer O, Meerwein H, Ziegler K. Stickstoffverbindungen I. In: Müller E, editor. Methoden der organischen Chemie (Houben-Weyl) 4th ed. Stuttgart, Germany: Georg Thieme Verlag; 1965. pp. 339–341.
-
- Dangmann E, Stolz A, Kuhm A E, Hammer A, Feigel B, Noisommit-Rizzi N, Rizzi M, Reuß M, Knackmuss H-J. Degradation of 4-aminobenzenesulfonate by a two-species bacterial coculture. Physiological interactions between Hydrogenophaga palleronii S1 and Agrobacterium radiobacter S2. Biodegradation. 1996;7:223–229. - PubMed
-
- Feigel B J, Knackmuss H-J. Bacterial catabolism of sulfanilic acid via catechol-4-sulfonate. FEMS Microbiol Lett. 1988;55:113–118.
-
- Feigel B J, Knackmuss H-J. Syntrophic interactions during degradation of 4-aminobenzenesulfonic acid by a two-species bacterial culture. Arch Microbiol. 1993;159:124–130. - PubMed
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

