D-Alanylcardiolipin, a major component of the unique lipid pattern of Vagococcus fluvialis
- PMID: 9603887
- PMCID: PMC107264
- DOI: 10.1128/JB.180.11.2950-2957.1998
D-Alanylcardiolipin, a major component of the unique lipid pattern of Vagococcus fluvialis
Abstract
Motile group N streptococci, classified as Vagococcus fluvialis, have been isolated from cows' udders, human and animal feces, river water, and seawater. They possess an unusual membrane lipid and fatty acid pattern. We isolated and characterized 13 polar lipids, 8 of them also found in other gram-positive bacteria: mono- and dihexosyldiacylglycerol, an acylated and a glycerophosphate-substituted derivative of the latter, cardiolipin, phosphatidylglycerol, D-alanylphosphatidylglycerol, and L-lysylphosphatidylglycerol. Besides them, we characterized two rare compounds, bis(acylglycero)phosphate and alpha-D-glucopyranosylcardiolipin, and two compounds so far not detected in nature, D-alanylbis(acylglycero)phosphate and D-alanylcardiolipin. The concomitant occurrence of four aminoacyl phospholipids in one organism is another unique finding. Substituted cardiolipins represent a novel lipid class: in vagococci, D-alanylcardiolipin is a major membrane lipid component, contributing 11 and 26 mol% of total lipids in the exponential and stationary phases of growth, respectively. The vagococcal lipids contain even-numbered straight-chain saturated and cis-monounsaturated fatty acids, but the cis-monoenic acids belong to the omega-9 series and not the omega-7 series, found in enterococci, lactococci, and streptococci.
Figures
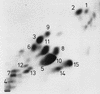
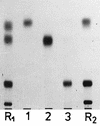

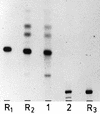


Similar articles
-
Polar lipids of four Listeria species containing L-lysylcardiolipin, a novel lipid structure, and other unique phospholipids.Int J Syst Bacteriol. 1999 Apr;49 Pt 2:653-62. doi: 10.1099/00207713-49-2-653. Int J Syst Bacteriol. 1999. PMID: 10408878
-
Teichoic acids and lipids associated with the membrane of a Bacillus licheniformis mutant and the membrane lipids of the parental strain.J Bacteriol. 1976 Oct;128(1):149-56. doi: 10.1128/jb.128.1.149-156.1976. J Bacteriol. 1976. PMID: 977537 Free PMC article.
-
Membrane phospholipid composition of Caulobacter crescentus.J Bacteriol. 1978 Sep;135(3):1130-6. doi: 10.1128/jb.135.3.1130-1136.1978. J Bacteriol. 1978. PMID: 690071 Free PMC article.
-
Amino acid-containing membrane lipids in bacteria.Prog Lipid Res. 2010 Jan;49(1):46-60. doi: 10.1016/j.plipres.2009.08.002. Epub 2009 Aug 22. Prog Lipid Res. 2010. PMID: 19703488 Review.
-
Quantitative and positional analysis of fatty acids.Lab Res Methods Biol Med. 1984;10:77-131. Lab Res Methods Biol Med. 1984. PMID: 6390054 Review.
Cited by
-
Roles of tRNA in cell wall biosynthesis.Wiley Interdiscip Rev RNA. 2012 Mar-Apr;3(2):247-64. doi: 10.1002/wrna.1108. Epub 2012 Jan 19. Wiley Interdiscip Rev RNA. 2012. PMID: 22262511 Free PMC article. Review.
-
Comparative genome analysis of Vagococcus fluvialis reveals abundance of mobile genetic elements in sponge-isolated strains.BMC Genomics. 2022 Aug 25;23(1):618. doi: 10.1186/s12864-022-08842-9. BMC Genomics. 2022. PMID: 36008774 Free PMC article.
-
Staphylococcus aureus resistance to human defensins and evasion of neutrophil killing via the novel virulence factor MprF is based on modification of membrane lipids with l-lysine.J Exp Med. 2001 May 7;193(9):1067-76. doi: 10.1084/jem.193.9.1067. J Exp Med. 2001. PMID: 11342591 Free PMC article.
-
Lysyl-Phosphatidylglycerol: A Lipid Involved in the Resistance of Staphylococcus aureus to Antimicrobial Peptide Activity.Antibiotics (Basel). 2025 Mar 28;14(4):349. doi: 10.3390/antibiotics14040349. Antibiotics (Basel). 2025. PMID: 40298503 Free PMC article. Review.
-
Isolation of glucocardiolipins from Geobacillus stearothermophilus NRS 2004/3a.J Bacteriol. 2002 Dec;184(23):6709-13. doi: 10.1128/JB.184.23.6709-6713.2002. J Bacteriol. 2002. PMID: 12426359 Free PMC article.
References
-
- Baer E, Kates M. Migration of esters of glycerophosphoric acid. II. The acid and alkaline hydrolysis of l-α-lecithins. J Biol Chem. 1950;185:615–623. - PubMed
-
- Behr T, Fischer W, Peter Katalinic J, Egge H. The structure of pneumococcal lipoteichoic acid. Improved preparation, chemical and mass spectrometric studies. Eur J Biochem. 1992;207:1063–1075. - PubMed
-
- Benson A A, Miyano M. The phosphatidylglycerol and sulfolipid of plants: asymmetry of the glycerol moiety. Biochem J. 1961;81:31. p.
-
- Beutler H O. Lactose and galactose. In: Bergmeyer H U, Bergmeyer J, Grassl M, editors. Methods of enzymatic analysis. 3rd ed. VI. Metabolites 1: carbohydrates. Weinheim, Germany: Verlag Chemie; 1984. pp. 104–112.
-
- Brotherus J, Renkonen O, Herrmann J, Fischer W. Novel stereoconfiguration in lyso-bis-phosphatidic acid of cultured BHK-cells. Chem Phys Lipids. 1974;13:178–182. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

