Localization of Mad2 to kinetochores depends on microtubule attachment, not tension
- PMID: 9606210
- PMCID: PMC2137189
- DOI: 10.1083/jcb.141.5.1181
Localization of Mad2 to kinetochores depends on microtubule attachment, not tension
Abstract
A single unattached kinetochore can delay anaphase onset in mitotic tissue culture cells (Rieder, C.L., A. Schultz, R. Cole, G. Sluder. 1994. J. Cell Biol. 127:1301-1310). Kinetochores in vertebrate cells contain multiple binding sites, and tension is generated at kinetochores after attachment to the plus ends of spindle microtubules. Checkpoint component Mad2 localizes selectively to unattached kinetochores (Chen, R.-H., J.C. Waters, E.D. Salmon, and A.W. Murray. 1996. Science. 274:242-246; Li, Y., and R. Benezra. Science. 274: 246-248) and disappears from kinetochores by late metaphase, when chromosomes are properly attached to the spindle. Here we show that Mad2 is lost from PtK1 cell kinetochores as they accumulate microtubules and re-binds previously attached kinetochores after microtubules are depolymerized with nocodazole. We also show that when kinetochore microtubules in metaphase cells are stabilized with taxol, tension at kinetochores is lost. The phosphoepitope 3f3/2, which has been shown to become dephosphorylated in response to tension at the kinetochore (Nicklas, R.B., S.C. Ward, and G.J. Gorbsky. 1995. J. Cell Biol. 130:929-939), is phosphorylated on all 22 kinetochores after tension is reduced with taxol. In contrast, Mad2 only localized to an average of 2.6 out of the 22 kinetochores in taxol-treated PtK1 cells. Therefore, loss of tension at kinetochores occupied by microtubules is insufficient to induce Mad2 to accumulate on kinetochores, whereas unattached kinetochores consistently bind Mad2. We also found that microinjecting antibodies against Mad2 caused cells arrested with taxol to exit mitosis after approximately 12 min, while uninjected cells remained in mitosis for at least 6 h, demonstrating that Mad2 is necessary for maintenance of the taxol-induced mitotic arrest. We conclude that kinetochore microtubule attachment stops the Mad2 interactions at kinetochores which are important for inhibiting anaphase onset.
Figures
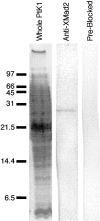

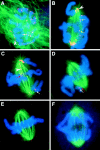






References
-
- Cassimeris L, Rieder CL, Rupp G, Salmon ED. Stability of microtubule attachment to metaphase kinetochores in PtK1 cells. J Cell Sci. 1990;96:9–15. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

