An alternate pathway for T cell development supported by the bone marrow microenvironment: recapitulation of thymic maturation
- PMID: 9607922
- PMCID: PMC2212319
- DOI: 10.1084/jem.187.11.1813
An alternate pathway for T cell development supported by the bone marrow microenvironment: recapitulation of thymic maturation
Abstract
In the principal pathway of alpha/beta T cell maturation, T cell precursors from the bone marrow migrate to the thymus and proceed through several well-characterized developmental stages into mature CD4+ and CD8+ T cells. This study demonstrates an alternative pathway in which the bone marrow microenvironment also supports the differentiation of T cell precursors into CD4+ and CD8+ T cells. The marrow pathway recapitulates developmental stages of thymic maturation including a CD4+CD8+ intermediary cell and positive and negative selection, and is strongly inhibited by the presence of mature T cells. The contribution of the marrow pathway in vivo requires further study in mice with normal and deficient thymic or immune function.
Figures


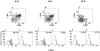


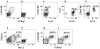
References
-
- Adkins B, Mueller C, Okada CY, Reichert RA, Weissman IL, Spangrude GJ. Early events in T-cell maturation. Annu Rev Immunol. 1987;5:325–365. - PubMed
-
- Weissman IL. Developmental switches in the immune system. Cell. 1994;76:207–218. - PubMed
-
- Shortman K. Cellular aspects of early T-cell development. Curr Opin Immunol. 1992;4:140–146. - PubMed
-
- Antica M, Wu L, Shortman K, Scollay R. Intrathymic lymphoid precursor cells during fetal thymus development. J Immunol. 1993;151:5887–5895. - PubMed
-
- Ismaili J, Antica M, Wu L. CD4 and CD8 expression and T cell antigen receptor gene rearrangement in early intrathymic precursor cells. Eur J Immunol. 1996;26:731–737. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

