The role of the thymus and recent thymic migrants in the maintenance of the adult peripheral lymphocyte pool
- PMID: 9607924
- PMCID: PMC2212318
- DOI: 10.1084/jem.187.11.1839
The role of the thymus and recent thymic migrants in the maintenance of the adult peripheral lymphocyte pool
Abstract
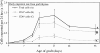
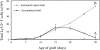
The thymus is essential for the initial seeding of T cells to the periphery, but its role in maintaining the adult T cell pool remains poorly defined. We investigated whether changes to the rate of T cell export could form part of the mechanism(s) controlling the homeostatic regulation of the size and composition of the peripheral T cell pool. Using neonatal thymi grafted under the kidney capsule, we found that irrespective of whether the pool was oversupplied (by thymic grafts) or undersupplied (due to neonatal thymectomy), the thymic export rate was constant from both the host and graft thymus, and the periphery remained constant in size. Recent thymic emigrants (RTE) were also tracked to determine the extent of their acceptance into the T cell pool of a normal mouse. As a population, RTE are phenotypically mature, but were distinct from resident T cells in the periphery, being released in a CD4/CD8 ratio approximately twice that of established peripheral T cells. This export ratio is similar to that of T cells in the mature thymic compartment, but soon after entry into the periphery, the ratio falls, indicating separate thymic and peripheral regulation of the CD4/CD8 ratio. RTE may also be preferentially incorporated into the periphery, causing displacement of resident T cells, thus maintaining the size of the peripheral pool. Although not vital for the maintenance of a functional T cell pool, the acceptance of RTE in a "full" peripheral pool would ensure that the T cell receptor repertoire is kept diverse and that the T cell population encompasses a broad range of naive as well as memory T cells.
Figures

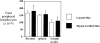



References
-
- Rocha B, Dautigny N, Pereira P. Peripheral T lymphocytes: expansion potential and homeostatic regulation of pool sizes and CD4/CD8 ratios in vivo. Eur J Immunol. 1989;19:905–911. - PubMed
-
- Grossman CJ. Interactions between the gonadal steroids and the immune system. Science. 1985;227:257–261. - PubMed
-
- Dardenne M, Savino W. Control of thymus physiology by peptidic hormones and neuropeptides. Immunol Today. 1994;15:518–523. - PubMed
-
- Scollay R, Butcher EC, Weissman IL. Thymus cell migration. Quantitative aspects of cellular traffic from the thymus to the periphery in mice. Eur J Immunol. 1980;10:210–218. - PubMed
-
- Tough DF, Sprent J. Lymphocyte life-span and memory. Science. 1994;265:1395–1400. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

