Thalidomide costimulates primary human T lymphocytes, preferentially inducing proliferation, cytokine production, and cytotoxic responses in the CD8+ subset
- PMID: 9607928
- PMCID: PMC2212313
- DOI: 10.1084/jem.187.11.1885
Thalidomide costimulates primary human T lymphocytes, preferentially inducing proliferation, cytokine production, and cytotoxic responses in the CD8+ subset
Abstract
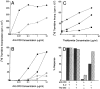
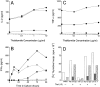
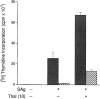
The efficacy of thalidomide (alpha-phthalimido-glutarimide) therapy in leprosy patients with erythema nodosum leprosum is thought to be due to inhibition of tumor necrosis factor alpha. In other diseases reported to respond to thalidomide, the mechanism of action of the drug is unclear. We show that thalidomide is a potent costimulator of primary human T cells in vitro, synergizing with stimulation via the T cell receptor complex to increase interleukin 2-mediated T cell proliferation and interferon gamma production. The costimulatory effect is greater on the CD8+ than the CD4+ T cell subset. The drug also increases the primary CD8+ cytotoxic T cell response induced by allogeneic dendritic cells in the absence of CD4+ T cells. Therefore, human T cell costimulation can be achieved pharmacologically with thalidomide, and preferentially in the CD8+ T cell subset.
Figures


References
-
- Sheskin J. Thalidomide in the treatment of lepra reactions. Clin Pharmacol Ther. 1965;6:303–306. - PubMed
-
- Sampaio EP, Kaplan G, Miranda A, Nery JA, Miguel CP, Viana SM, Sarno EN. The influence of thalidomide on the clinical and immunologic manifestation of erythema nodosum leprosum. J Infect Dis. 1993;168:408–414. - PubMed
-
- Waters MFR. An internally controlled double-blind trial of thalidomide in severe erythema nodosum leprosum. Lepr Rev. 1971;42:26–42. - PubMed
-
- Jacobson JM, Greenspan JS, Spritzler J, Ketter N, Fahey JL, Jackson JB, Fox L, Chernoff M, Wu AW, MacPhail LA, et al. Thalidomide for the treatment of oral aphthous ulcers in patients with human immunodeficiency virus infection. N Engl J Med. 1991;336:1487–1493. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

