Functional reconstitution of a prokaryotic K+ channel
- PMID: 9607934
- PMCID: PMC2217152
- DOI: 10.1085/jgp.111.6.741
Functional reconstitution of a prokaryotic K+ channel
Abstract
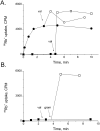
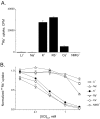
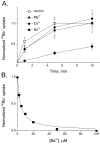
SliK, a K+ channel encoded by the Streptomyces KcsA gene, was expressed, purified, and reconstituted in liposomes. A concentrative 86Rb+ flux assay was used to assess the ion transport properties of SliK. SliK-mediated ionic flux shows strong selectivity for K+ over Na+ and is inhibited by micromolar concentrations of Ba2+, mirroring the basic permeation characteristic of eukaryotic K+ channels studied by electrophysiological methods. 86Rb+ uptake kinetics and equilibrium measurements also demonstrate that the purified protein is fully active.
Figures
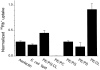

References
-
- Cortes DM, Perozo E. Structural dynamics of the Streptomyces lividans K+ channels (SCK+): oligomeric stoichiometry and stability. Biochemistry. 1997;36:10343–10352. - PubMed
-
- Cuello LG, Romero JG, Cortes DM, Perozo E. pH-dependent gating in the Streptomyces lividans K+channel. Biochemistry. 1998;37:3229–3236. - PubMed
-
- Doyle DA, Cabral JM, Pfuetzner A, Kuo JM, Gulbis JM, Cohen SL, Chait BT, MacKinnon R. The structure of the potassium channel: molecular basis of K+conduction and selectivity. Science. 1998;280:69–76. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

